Malaysian Journal of Analytical
Sciences, Vol 26
No 5 (2022): 976 - 988
MICROBIAL DEGRADATION OF PALM OIL IN
NATURAL SEAWATER AND IDENTIFICATION OF OIL DEGRADING BACTERIAL CONSORTIUM
(Degradasi Mikrob Minyak Sawit dalam
Air Laut Semula Jadi dan Pengenalpastian Konsortium Bakteria Pendegradasi Minyak)
Arularasu
Muthaliar Tamothran1,2, Sree Selva Kumar Ganesen1, Hing Lee Siang1,
Sabiqah Tuan Anuar1,
Razmah
Ghazali2, Kesaven Bhubalan1,2,3*
1Faculty
of Science and Marine Environment,
Universiti Malaysia Terengganu, 21030 Kuala Nerus,
Terengganu, Malaysia
2Malaysian
Palm Oil Board (MPOB),
Persiaran Institusi, Bandar Baru Bangi, 43000 Kajang
Selangor, Malaysia
3Institute
of Marine Biotechnology (IMB),
Universiti Malaysia Terengganu, 21030 Kuala Nerus,
Terengganu, Malaysia
*Corresponding
author: kesaven@umt.edu.my
Received: 15 March 2022; Accepted: 3
July 2022; Published: 30 October 2022
Abstract
Palm oil industry is
among the most important commodities industry in Malaysia where Malaysia
dominates 39% and 44% of global palm oil production and exports respectively.
Most of the palm oil exports to various countries are done via sea-shipping
which increases the risk towards marine pollution in form of oil spillage from
vessels. Microbial degradation studies are important in establishing baseline
data which is instrumental for mitigation planning and policy making.
Degradation of palm oil derivatives was investigated using natural seawater
collected from Klang Port and isolated bacteria Pseudomonas
aeruginosa UMTKB-5 based on modified shake flask method as described by
OECD Guidelines for Testing Chemicals, OECD TG 306 (Biodegradability in
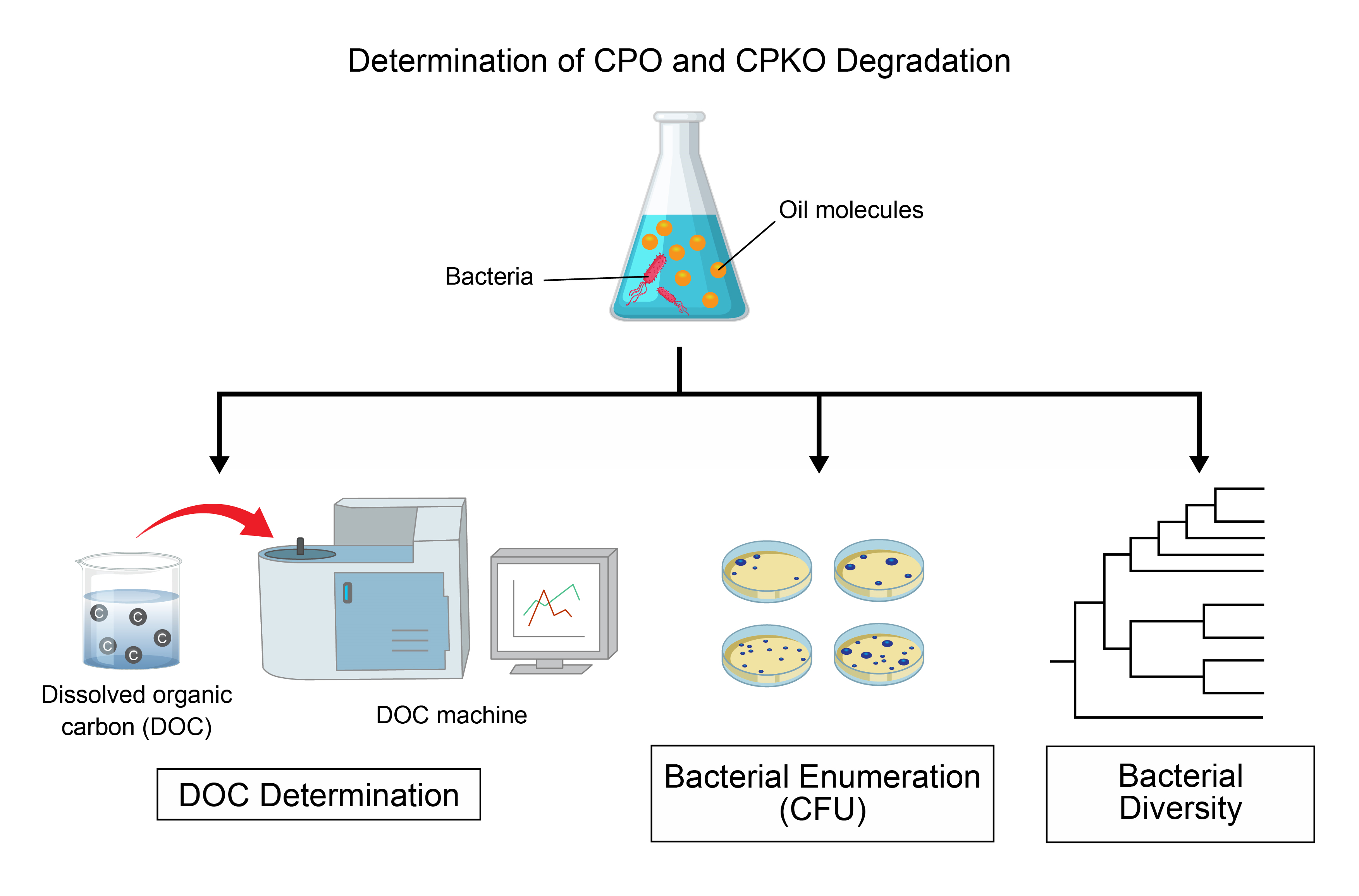
Seawater). Analytical method for determination of CPO and CPKO degradation
comprises of measurement of dissolved organic carbon (DOC), colony forming unit
(CFU), bacterial diversity using 16S rDNA gene based metagenomic analysis, and
fatty acid measurements. Degradation of CPO and CPKO in seawater collected from
Klang Port show increase in bacterial population
which peaked in day 7 and 21 before declining indicating that palm oil is being
used as substrate for bacterial growth in tandem with degradation which is
aided by lipase enzyme produced by selected bacteria. Similar growth pattern
observed in P. aeruginosa UKTKB-5 cultivated sample. DOC removal in
sample showed negative value showing that carbon input from CPO and CPKO
degradation is higher than consumption by bacteria. Fatty acid measurement
shows changes in composition where bacterial degradation and utilisation of oil.
The metagenomic analysis revealed diverse bacteria population in the different
sampling locations and four lipase-producing bacterial strains were isolated at
the end of the biodegradation experiment. The study has shown the
biodegradability of palm oil in seawater and able to provide baseline data to
understand and formulate the action plan in the event of spill in marine
environment.
Keywords:
palm oil, bacteria diversity,
microbial degradation, fatty acids
Abstrak
Industri minyak sawit
adalah antara industri komoditi terpenting di Malaysia di mana Malaysia
menguasai 39% dan 44% pengeluaran dan eksport minyak sawit secara global
masing-masing. Kebanyakan eksport minyak sawit ke pelbagai negara dilakukan
melalui perkapalan laut yang meningkatkan risiko pencemaran marin dalam bentuk
tumpahan minyak dari kapal. Kajian degradasi mikrob
yang mampan adalah penting dalam mengesahkan data
asas yang memainkan peranan penting untuk perancangan mitigasi
dan pembuatan dasar dan polisi. Degradasi minyak
sawit tidak ditapis (CPO) dan minyak isirong sawit
tidak ditapis (CPKO) dalam air laut semula jadi yang dikumpul
dari Pelabuhan Klang and bakteria Pseudomonas
aeruginosa UMTKB-5 telah dikaji menggunakan
kaedah kelalang goncang yang diubah suai seperti yang diterangkan oleh garis
panduan OECD untuk pengujian bahan kimia, OECD TG 306 (kebolehbiodegradan
dalam air laut). Analisa process
degradasi CPO and CPKO
melibatkan pengukuran karbon organik terlarut (DOC), kiraan unit pembentuk koloni
(CFU), analisis metagenomik berasaskan gen 16S rDNA untuk kepelbagaian bakteria, dan perubahan acid lemak bebas. Keputusan menunjukkan peningkatan dalam
kiraan CFU apabila bilangan hari meningkat dan kiraan CFU berada pada tahap
tertinggi pada hari ke-7 dan 21 sebelum menurun. Situasi ini menunjukkan
penggunaan minyak sawit sebagai substrat oleh
bakteria. Peningkatan populasi bakteria yang sama ditunjukkan oleh bakteri P. aeruginosa UMTKB-5.
Perubahan nilai penyingkiran DOC kepada nilai negatif mendedahakan
kemasukan karbon melalui process degradasi
CPO and CPKO pada tahap yang melebihi penggunaan oleh
bakteria. Perubahan komposisi asid lemak penggunaan substrat
oleh bakteria. Analisis metagenomik mendedahkan
populasi bakteria yang pelbagai di lokasi persampelan
yang berbeza dan lebih daripada empat strain bakteria
telah diasingkan pada akhir eksperimen biodegradasi. Strain positif juga diuji untuk aktiviti lipolitik. Kajian ini telah mendedahkan kebolehbiodegradasian
minyak sawit dalam air laut dan dijangka dapat membekalkan maklumat asas kepada
pihak berkuasa berkaitan minyak sawit, pengangkutan dan agensi alam sekitar
untuk memahami dan merangka pelan tindakan sekiranya berlaku tumpahan dalam
persekitaran marin.
Kata
kunci: minyak sawit, kepelbagaian bakteria, bakteria pendegradasi, asid lemak

References
1.
El-Hamidi, M. and
Zaher, F. A. (2018). Production of vegetable oils in the world and in Egypt: An
overview. Bulletin of the National Research Centre, 42(1): 19.
2.
USDA. (2017). United
States Department of Agriculture. Accessed at www.fas.usda.gov
[Access date March 01, 2022].
3.
Purba,
H. J., Sinaga, B. M., Novianti,
T. and Kustiari, R. (2018). The impact of changes in
external factors on the world vegetable oil market. International Journal of
Economics and Financial Issues, 8(6): 176.
4.
MPOB. (2017). Pocketbook
of Oil Palm Statistics. Malaysia, Malaysian Palm Oil Board.
5.
Sambanthamurthi,
R., Sundram, K. and Tan, Y. A. (2000). Chemistry and
biochemistry of palm oil. Progress in Lipid Research, 39(6): 507-558.
6.
Bucas,
G., and Saliot, A. (2002). Sea transport of animal
and vegetable oils and its environmental consequences. Marine Pollution
Bulletin, 44(12): 1388-1396.
7.
South China Morning
Post (2017). Japanese-owned tanker and Singapore container ship identified as
vessels in collision leading to palm oil spill. Accessed from http://www.scmp.com/news/hong-kong/health-environment/article/2106702/japanese-owned-tanker-and-singapore-container-ship
[Access date March 01, 2022].
8.
The Telegraph. (2018).
Ships dumping noxious palm oil off British coast without legal consequences.
Accessed from https://www.telegraph.co.uk/news/2018/02/03/ships-dumping-noxious-palm-oil-british-coast-without-legal-consequences
[Access date March 01, 2022].
9.
USEPA. (2015).
Emergency response, oil spills prevention and preparedness regulations -
vegetable oils and animal fats. Accessed from http://www.epa.gov/emergency-response/vegetable-oils-and-animal-fats
[Access date March 01, 2022].
10.
APHA. (1999). Standard
Methods of Water and Wastewater. Washington D.C., USA, American Public Health
Association.
11.
OECD. (1992). Test No. 306: Biodegradability in seawater.
Paris, OECD Publishing. Accessed date March 01, 2022]
12.
Karanfil,
T., Erdogan, I. and Schlautman, M. A. (2003).
Selecting filter membranes for measuring DOC and UV254. Journal - American
Water Works Association, 95(3): 86-100.
13.
Suratman,
S., Zan, N. H. C., Aziz, A. A. and Tahir, N. M. (2017). Spatial and seasonal
variations of organic carbon-based nutrients in Setiu
wetland, Malaysia. Sains Malaysiana, 46(6):
859-865.
14.
Suratman,
S., Weston, K., Jickells, T. and Fernand, L. (2009).
Spatial and seasonal changes of dissolved and particulate organic C in the
North Sea. Hydrobiologia, 628(1): 13-25.
15.
Bhubalan,
K., Hui-Wan, R. A. C., Renganathan, P., Tamothran, A. M., Ganesen, S. S.
K. and Ghazali, R. (2019). Bacterial degradation of palm olein in seawater and
identification of some cultivable strains. Malaysian Applied Biology,
48(1): 1-7.
16.
Hongoh,
Y., Yuzawa, H., Ohkuma, M. and Kudo, T. (2003).
Evaluation of primers and PCR conditions for the analysis of 16S rRNA genes
from a natural environment. FEMS Microbiology Letters, 221(2): 299-304.
17.
Azran,
M. F. M., Fazielawanie, N., Saidin,
J., Wahid, E. and Bhubalan, K. (2014). Bioconversion
of cane sugar refinery by-products into rhamnolipid by marine Pseudomonas
aeruginosa. Proceedings of International Conference on Beneficial
Microbes, 2014: 259-262.
18.
Shahaliyan,
F., Safahieh, A. and Abyar,
H. (2015). Evaluation of emulsification index in marine bacteria Pseudomonas
sp. and Bacillus sp. Arabian Journal for Science and Engineering,
40(7): 1849-1854.
19.
Bhubalan,
K., Rathi, D. N., Abe, H., Iwata, T. and Sudesh, K. (2010). Improved synthesis
of P (3HB-co-3HV-co-3HHx) terpolymers by mutant Cupriavidus necator
using the PHA synthase gene of Chromobacterium
sp. USM2 with high affinity towards 3HV. Polymer Degradation and Stability,
95(8): 1436-1442.
20.
Anuar,
S. T., Mugo, S. M. and Curtis, J. M. (2015). A
flow-through enzymatic microreactor for the rapid conversion of
triacylglycerols into fatty acid ethyl ester and fatty acid methyl ester
derivatives for GC analysis. Analytical Methods, 7(14): 5898-5906.
21.
Russell, D. J. and
Carlson, B. A. (1978). Edible-oil pollution on Fanning Island. Pacific
Science, 32(1): 1-15.
22.
Mudge,
S. M. (1995). Deleterious effects from accidental spillages of vegetable oils. Spill
Science & Technology Bulletin, 2(2-3): 187-191.
23.
Ott, A., Martin, T. J.,
Whale, G. F., Snape, J. R., Rowles, B., Galay-Burgos,
M. and Davenport, R. J. (2019). Improving the biodegradability in seawater test
(OECD 306). Science of the Total Environment, 666: 399-404.
24.
Al-Darbi,
M. M., Saeed, N. O., Islam, M. R. and Lee, K. (2005). Biodegradation of natural
oils in seawater. Energy sources, 27(1-2): 19-34.
25.
Mudge,
S. M., Salgado, M. A. and East, J. (1993). Preliminary investigations into
sunflower oil contamination following the wreck of the MV Kimya.
Marine Pollution Bulletin, 26(1): 40-44.
26.
Thouand,
G., Friant, P., Bois, F., Cartier, A., Maul, A. and
Block, J. C. (1995). Bacterial inoculum density and probability of
para-nitrophenol biodegradability test response. Ecotoxicology and
Environmental Safety, 30(3): 274-282.
27.
Marsudi,
S., Unno, H. and Hori, K. (2008). Palm oil
utilization for the simultaneous production of polyhydroxyalkanoates
and rhamnolipids by Pseudomonas aeruginosa. Applied Microbiology and
Biotechnology, 78(6): 955-961.
28.
Thaniyavarn,
J., Chongchin, A., Wanitsuksombut,
N., Thaniyavarn, S., Pinphanichakarn,
P., Leepipatpiboon, N., Morikawa, M. and Kanaya, S. (2006). Biosurfactant production by Pseudomonas
aeruginosa A41 using palm oil as carbon source. The Journal of General
and Applied Microbiology, 52(4): 215-222.
29.
Karlapudi,
A. P., Venkateswarulu, T. C., Tammineedi,
J., Kanumuri, L., Ravuru,
B. K., Dirisala, V. and Kodali,
V. P. (2018). Role of biosurfactants in bioremediation of oil pollution - A
review. Petroleum, 4(3): 241-249.