Malaysian Journal of Analytical
Sciences, Vol 26
No 5 (2022): 1070 - 1081
OPTIMIZATION
OF MICROWAVE-ASSISTED EXTRACTION OF PHENOLIC COMPOUNDS FROM Eleusine indica USING RESPONSE SURFACE
METHODOLOGY
(Pengoptimuman Pengekstrakan Berbantu Gelombang Mikro bagi
Sebatian Fenolik dari Eleusine indica Mengunakan Kaedah Gerak Balas Permukaan)
Angelica A. Angeles-Macalalad,
Bryan John A. Magoling*, Jennielyn C. De Chavez,
Love Angel H. Flores, Alona B. Intac
Department of Chemistry,
College of Arts and Sciences,
Batangas State University-Pablo Borbon, Rizal Avenue,
Batangas City, 4200 Philippines
*Corresponding author: bryanmagoling@gmail.com
Received: 28 February 2022; Accepted:
19 May 2022; Published: 30 October 2022
Abstract
Eleusine indica belongs to the Pocaceae
family and is abundantly found in many tropical countries. Because of its
anti-malarial, antioxidant, anti-viral, and antidiabetic properties, among many
others, studies are being aimed on developing time and cost-effective methods
that could efficiently extract its active components. This study focused on the
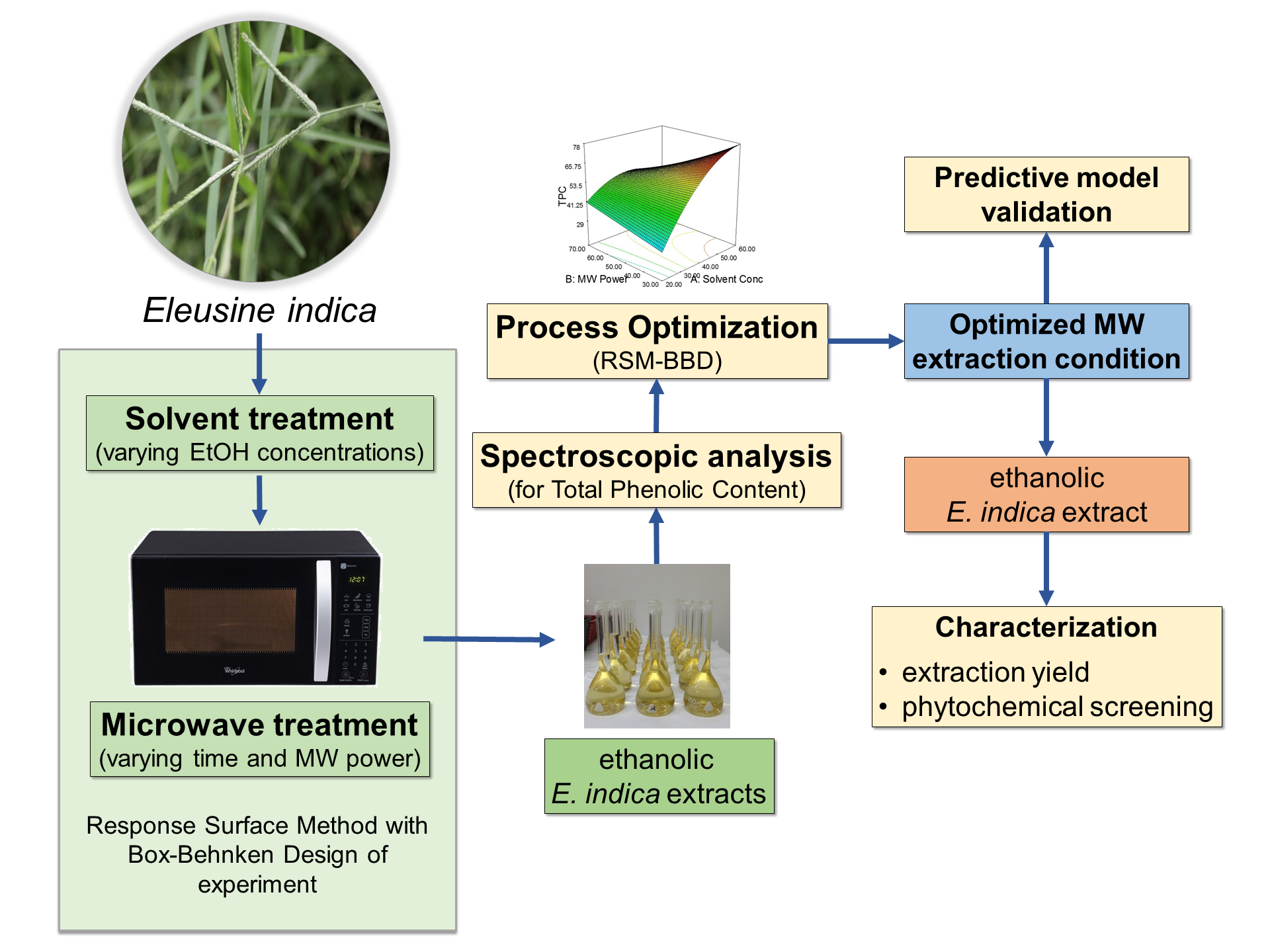
optimization of the microwave-assisted extraction (MAE) of phenolic contents
from E. indica. A response surface
methodology (RSM) using a Box-Behnken design (BBD) of experiment was employed
to determine the optimized condition for the extraction method. The extraction
was performed with three varying factors (ethanol concentration, microwave
power, and irradiation time) and one response (total phenolic content or TPC).
A maximum TPC of 74.81 ± 5.22 GAE mg/g was obtained through MAE, using 57.23%
(v/v) ethanol with microwave irradiation of 217.77 W for 4.53 minutes. The
optimized condition had an extraction yield of 11.62 ± 1.97%. The ethanolic E. indica extract obtained using the
optimum condition contained mostly of triterpenes, saponins and glycosides; and
moderately of flavonoids and tannins.
Keywords:
Eleusine indica, microwave-assisted
extraction, phenolic compound extraction, response surface methodology
Abstrak
Eleusine indica dari
keluarga Pocaceae
dan ia sering dijumpai di negara tropika. Oleh kerana sifatnya anti-malaria,
antioksida, anti-viral dan antidiabetik, banyak kajian dijalankan bangi
membangunkan kaedah yang berkesan dari aspek masa dan kos bagi tujuan
pengkestrakan komponen aktif. Kajian ini memberi tumpuan kepada pengoptimuman
pengekstrakan berbantu gelombang mikro (MAE) bagi kandungan fenolik dari E. indica. Kaedah gerak balas permukaan
(RSM) mengunakan reka bentuk eksperimen Box-Behnken telah dibangunkan bagi
penentuan keadaan optimum kaedah pengekstrakan. Pengekstrakan telah dijalankan
Bersama tiga faktor (kepekatan etanol, kuasa gelombang mikro, dan masa
penyinaran) dan satu respons (jumlah kandungan fenolik atau TPC). Nilai
maksimum TPC ialah 74.81 ± 5.22 GAE mg/g diperolehi melalui MAE, mengunakan
57.23% (v/v) etanol bersama 217.77 W penyinaran gelombang mikro selama 4.53
minit. Keadaan optimum telah
menghasilkan ekstrak 11.62 ± 1.97%. Ekstrak etanolik E. indica mengunakan
keadaan optimum mengandungi triterpen, saponin dan glikosida, dan flavonoid dan
tannin.
Kata kunci: Eleusine indica, pengekstrakan berbantu gelombang mikro, pengekstrakan sebatian fenolik,
kaedaj gerak balas permukaan
References
1.
Quiroga,
E., Sampietro, A. and Vattuone,
M. (2001). Screening antifungal activities of selected medicinal plants. Journal
of Ethnopharmacology, 74: 89-96.
2.
Zhang, H. (2016).
Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory
effects. Current Opinion in Food Science,
8: 33-42.
3.
Nile, S., Park, S. W.
(2013). Edible Berries: Bioactive components and their effect on human health.
Nutrition. Burbank, Los Angeles County, California, 30.
4.
Al-Zubairi,
A., Abdul, A., Abdelwahab,
S. I., Peng, C. Y. P., Mohan, S. and Elhassan, M. M. (2011). Eleucine indica Possesses Antioxidant, Antibacterial
and Cytotoxic Properties. Evidence-based Complementary and Alternative
Medicine. eCAM. p. 965370.
5.
Deng, H. and Dai, J.
(2009). Preparation and characterization of activated carbon from cotton stalk
by microwave assisted chemical activation—application in methylene blue
adsorption from aqueous solution. Journal
of Hazardous Materials, 166(2-3): 1514-1521.
6.
Del Rio, D., Borges,
G. and Crozier, A. (2010). Berry flavonoids and phenolics: Bioavailability and
evidence of protective effects. British
Journal of Nutrition, 3: S67-90.
7.
Chitindingu,
K., Chitindingu,
J. J., Benhura, M. A. N., Marume,
A., Mutingwende, I., Bhebhe,
M. and Muchuweti, M. (2012). Antioxidant
capacity of bioactive compounds extracted from selected wild and domesticated
cereals of Zimbabwe. African Journal of
Biochemistry Research, 6: 62-68.
8.
Iqbal, M. and Gnanaraj, C. (2012). Eleusine
indica L. possesses antioxidant activity and precludes carbon tetrachloride
(CCl4)-mediated oxidative hepatic damage in rats. Environmental Health and Preventive Medicine,
17(4): 307-315.
9.
Okokon,
J. (2010). Antiplasmodial and antidiabetic activities
of Eleusine indica. International Journal of Drug Development
and Research, 2: 493-500.
10. Gruyal,
G. (2014). Ethnomedicinal plants used by residents in Northern Surigao del Sur,
Philippines. Natural Products Chemistry
& Research, 20142-4.
11.
Iberahim,
R., Yaacob, A. and Ibrahim, N. (2015). Phytochemistry, cytotoxicity and
antiviral activity of Eleusine indica
(sambau). Proceedings
of the Universiti Kebangsaan
Malaysia, Faculty of Science and Technology 2015 Postgraduate Colloquium, 030013-01-030013-04.
12.
Kore K. J., Shete R. V. and Desai N. V. (2011). Anti-arthritic activity
of hydroalcoholic extract of Lawsonia Innermis. International
Journal of Drug Development and Research, 3: 217-224.
13. Morah
F. and Otuk M. (2015). Antimicrobial and antihelmintic activity of Eleusine indica. Acta
Scientiae et Intellectus, 1(4): 28-32.
14. Ignat
I, Volf I, Popa V. (2011). A critical review of
methods for characterisation of polyphenolic
compounds in fruits and vegetables. Food
Chemistry, 126(4): 1821-1835.
15. Wang,
L. (2006). Recent advances in extraction of nutraceuticals from plants. Trends in Food Science & Technology,
17(6): 300-312.
16.
Nor Halaliza A. and Zulkifly A.
(2017). Microwave-assisted extraction of phenolic compound from pineapple
skins: The optimum operating condition and comparison with Soxhlet extraction. Malaysian Journal of Analytical Sciences,
21(3): 690-699.
17.
Kan, X., Zhang, J.,
Tong, Y. W. and Wang C. H. (2018). Overall evaluation of microwave-assisted
alkali pretreatment for enhancement of biomethane production from brewers’
spent grain. Energy Conversion and
Management, 158: 315-326.
18.
Eskilsson
C. and Björklund E. (2001). Analytical-scale microwave-assisted extraction. Journal of Chromatography, 902: 227-250.
19.
Lundstedt, T., Seifert, E., Abramo,
L., Thelin, B., Nystrom, A., Pettersen, J. and
Bergman, R. (1998). Experimental design and optimization. Chemometrics and Intelligent Laboratory
Systems, 42(1): 3-40.
20.
Koffi,
E., Sea T., Dodehe, Y., and Soro,
S. (2010). Effect of solvent type on extraction of polyphenols from
twenty-three ivorian plants. Journal Animal Plant
Sciences, 5: 550-558.
21.
Randika, S., Nilushi, N., Pathmalal, M., Lanka, U., Dhanushka,
U. and Nilmini, L. (2021). Effects of extraction
solvents on phytochemical screening, cytotoxicity and anti-obesity activities
of selected Sri Lankan medicinal plants. Pharmacognosy Research, 13(4):
246-256.
22. Pan,
X, Niu, G. and Liu, H. (2003). Microwave-assisted
extraction of tea polyphenols and tea caffeine from green tea leaves. Chemical Engineering and Processing: Process
Intensification, 42(2): 129-133.
23.
Rezaei, S., Rezaei,
K., Haghighi, M. and Labbafi
M. (2013). Solvent and solvent to sample ratio as main parameters in the
microwave-assisted extraction of polyphenolic compounds from apple pomace. Food Science and Biotechnology, 22(5):
1-6.
24.
Zhang, S. Q., Bi, H.
M. and Liu, C. J. (2007). Extraction of bio-active components from Rhodiola sachalinensis
under ultrahigh hydrostatic pressure. Separation
and Purification Technology, 57(2): 277-282.
25.
Trease,
G. E. and Evans, W. C. (2002). A text book of pharmacognosy, 15th
edition. London: Academic press.
26.
Magoling,
B. J. A. and Macalalad, A. A. (2017). Optimization
and response surface modelling of activated carbon production from mahogany
fruit husk for removal of chromium (VI) from aqueous solution. BioResources, 12:
3001-3016.
27.
Kaderides,
K., Papaoikonomou, L., Serafim, M. and Goula, A. (2019). Microwave-assisted extraction of
phenolics from pomegranate peels: Optimization, kinetics, and comparison with
ultrasounds extraction. Chemical
Engineering & Processing: Process Intensification, 137: 1-11.
28.
Shang, A., Luo, M., Gan, R. Y., Xu, X., Xia, Y., Liu,
Y. and Li, H. B. (2020). Effects of microwave-assisted extraction
conditions on antioxidant capacity of sweet tea (Lithocarpus
polystachyus Rehd.). Antioxidants, 9(8): 678.
29.
Peng, F., Cheng, C., Xie, Y.
and Yang, Y. (2015). Optimization of microwave-assisted extraction of
phenolic compounds from “Anli” pear (Pyrus ussuriensis Maxim). Food
Science and Technology Research, 21(3): 463-471.
30. Zheng,
X., Xu, X., Liu, C., Sun, Y., Lin, Z. and Liu, H. (2013). Extraction
characteristics and optimal parameters of anthocyanin from blueberry powder
under microwave-assisted extraction conditions. Separation and Purification Technology, 104: 17-25.
31. Akhtar,
I., Javad, S., Yousaf, Z., Igbal, S. and Jabeen, K. (2019). Microwave assisted extraction of
phytochemicals an efficient and modern approach for botanicals and
pharmaceuticals. Pakistan Journal
Pharmaceutical Sciences, 32(1): 223-230.
32. Mandal,
V., Mohan, Y. and Hemalatha, S. (2007). Microwave
assisted extraction – an innovative and promising extraction tool for medicinal
plant research. Pharmacognosy Reviews,
1(1): 7-18.