Malaysian Journal of Analytical
Sciences, Vol 26
No 5 (2022): 924 - 935
SYNTHESIS, ELECTROCHEMICAL ANALYSIS
AND DFT CALCULATION OF NEW ALKOXYLATED-CHALCONE AS SEMICONDUCTOR MATERIAL
(Sintesis, Analisis Elektrokimia dan Pengiraan DFT bagi
Alkoksi-Kalkon
Sebagai Bahan Semikonduktor)
Syaharil
Saidin1, Wan M. Khairul1*, Rafizah Rahamathullah2
1Faculty of Science and Marine
Environment,
Universiti Malaysia Terengganu,
21030, Kuala Nerus, Terengganu, Malaysia
2Faculty of Engineering Technology,
Universiti Malaysia Perlis,
Level 1, Block S2, UniCITI Alam
Kampus, Sungai Chuchuh, Padang Besar, 02100 Perlis, Malaysia
*Corresponding
author: wmkhairul@umt.edu.my
Received: 13 March 2022; Accepted: 18
May 2022; Published: 30 October 2022
Abstract
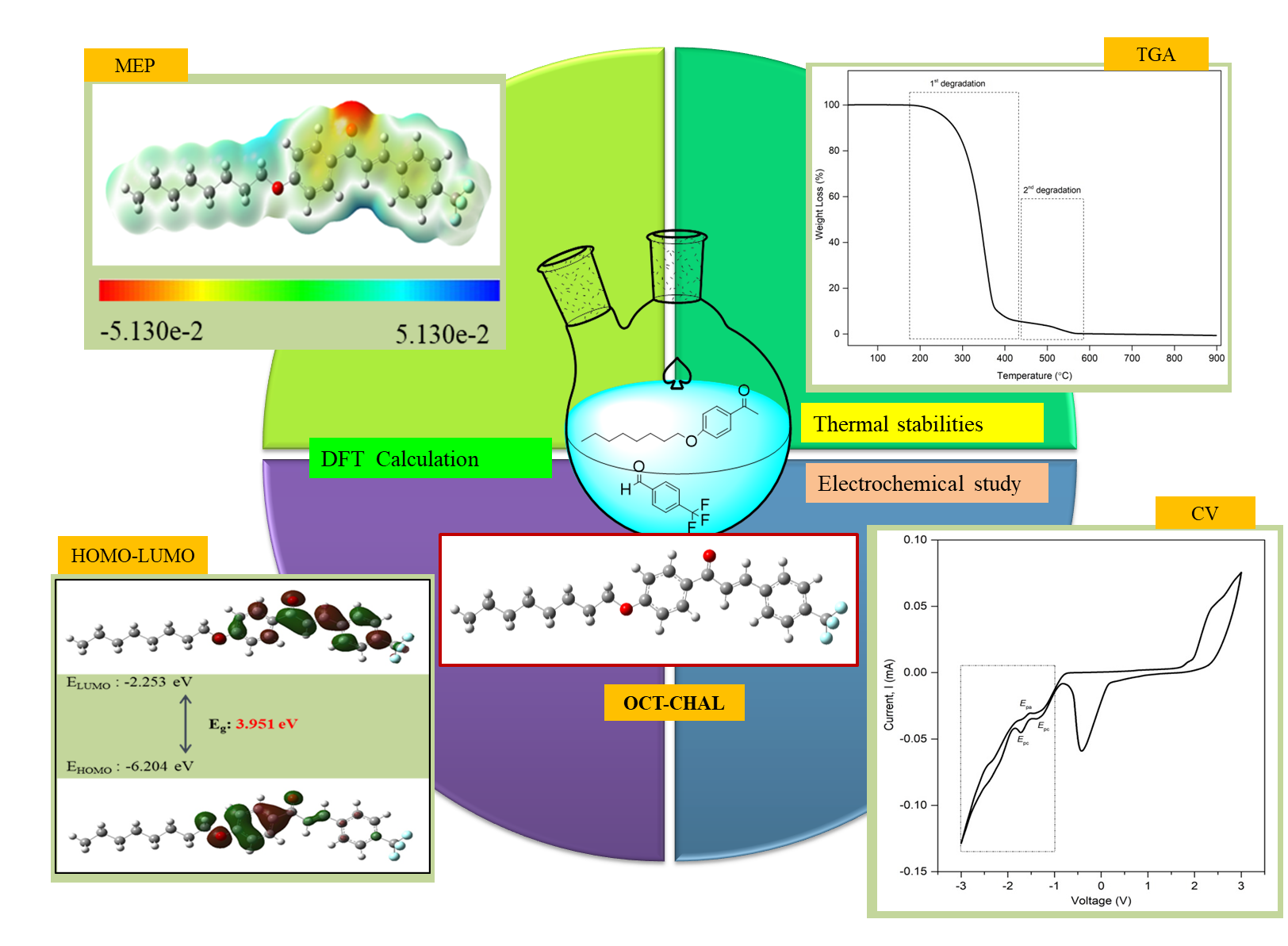
A new alkoxylated-chalcone derivative displaying donor(D)-p-acceptor(A) system was successfully synthesized via
Claisen-Schmidt condensation to be integrated as organic semiconductor
material. The correlation between electronic, optical properties,
electrochemical and DFT calculation of the designated system were assessed in
thorough. This alkoxylated-chalcone (OCT-CHAL) reveals an absorption band at lmax 312 nm with molar coefficient of ca. 105
L mol-1 cm-1 which corresponding to p-p* transition. The optical band
gap gives the value of 3.40 eV which shows good agreement with the value of
energy level between HOMO and LUMO for theoretical calculation. Meanwhile, this
derivative also reveals good thermal stability up to 200 ℃ with total mass loss at 97 %.
In turn, the electrochemical behaviour of OCT-CHAL
was investigated and exhibited redox potential with the values of 2.40 V
for oxidation potential while the reduction potential exhibited at value of
-1.72 V as quasi-reversible reduction. From the outcome, the existence of
conjugation effect on chalcone moiety with ideal relation between the
experimental and theoretical approach provides good support of being a
potential candidate in organic semiconductor material for advance material
application.
Keywords:
alkoxy,
chalcone, bandgap, DFT, electrochemistry
Abstrak
Terbitan alkoksi-kalkon
baharu yang memaparkan sistem penderma(D)-p-penerima(D) telah berjaya disintesis melalui kaedah
kondensasi Claisen-Schmidt untuk diintegrasikan sebagai bahan organik
semikonduktor. Perkaitan antara elektronik, sifat optik, elektrokimia dan
pengiraan DFT bagi sistem yang direkabentuk telah dinilai secara menyeluruh. Alkoksi-kalkon (OCT-CHAL) ini menunjukkan jalur serapan pada lmak 312 nm dengan
pekali molar 105 L mol-1 cm-1 yang selari
dengan peralihan p-p*. Nilai
jurang jalur optik memberikan nilai 3.40 eV yang mana keputusannya menunjukkan
nilai yang selari dengan nilai aras tenaga antara HOMO dan LUMO bagi pengiraan
teori. Sementara
itu, terbitan ini juga menunjukkan kestabilan terma yang baik sehingga 200
˚C dengan jumlah jisim yang hilang sebanyak 97 %. Selanjutnya sifat
elektrokimia bagi OCT-CHAL telah dikaji dan menunjukkan keupayaan redoks
dengan nilai 2.40 V untuk potensi pengoksidaan manakala potensi penurunan
adalah pada nilai -1.72 V sebagai penurunan kuasi-terbalik. Daripada keputusan
yang diperolehi, kehadiran kesan konjugasi pada moiti kalkon dengan hubungan
yang baik antara kaedah eksperimen dan teori memberikan sokongan yang baik
untuk menjadi calon berpotensi dalam bahan organik semikonduktor bagi aplikasi
bahan termaju.
Kata kunci: alkoksi, kalkon, jurang jalur, DFT,
Elektrokimia
References
1.
Chen, F. C. (2018). Organic
semiconductors. Encyclopedia of Modern Optics, Elsevier: pp.
220-231.
2.
Fayed, T. A. (2006). A novel
chalcone-analogue as an optical sensor based on ground and excited states
intramolecular charge transfer: A combined experimental and theoretical
study. Chemical Physics, 324 (2-3): 631-638.
3.
Bangal,
P. R., Lahiri, S., Kar, S. and Chakravorti,
S. (1996). Vibronic interaction and photophysics of
chalcone derivatives. Journal of Luminescence, 69(1): 49-56.
4.
Kagatikar,
S. and Sunil, D. (2021). Aggregation induced emission of chalcones. Chemical
Papers, 75(12):
6147-6156.
5.
Coskun, D., Gunduz, B. and Coskun, M. F. (2019). Synthesis,
characterization and significant optoelectronic parameters of
1-(7-methoxy-1-benzofuran-2-yl) substituted chalcone derivatives. Journal
of Molecular Structure, 1178:
261-267.
6.
Nappi, C., Romeo, F. and Sarnelli, E. (2020). Electronic properties of
one-dimensional pentacene crystals. Nano Express, 1 (3): 030002.
7.
da Costa, R. G., Farias, F.
R., Maqueira, L., Castanho,
C., Carneiro, L. S., Almeida, J., Buarque, C., Aucélio,
R. and Limberger, J. (2019). Synthesis, photophysical
and electrochemical properties of novel D-π-D and D-π-A triphenylamino-chalcones and β-arylchalcones. Journal
of the Brazilian Chemical Society, 30(1): 81-89.
8.
Lee, S. T., Khairul, W. M.,
Lee, O. J., Rahamathullah, R., Daud, A. I., KuBulat, K. H., Sapari, S.,
Razak, F. I. A. and Krishnan, G. (2021).
Electronic, reactivity and third order nonlinear optical properties of
thermally-stable push-pull chalcones for optoelectronic interest: experimental
and DFT assessments. Journal of Physics and Chemistry of Solids, 159: 110276.
9.
Makhlouf,
M. M., Radwan, A. S. and Ghazal, B. (2018). Experimental and DFT insights into
molecular structure and optical properties of new chalcones as promising
photosensitizers towards solar cell applications. Applied Surface
Science, 452: 337-351.
10. Namal, I., Ozelcaglayan, A. C., Udum, Y. A.
and Toppare, L. (2013). Synthesis and electrochemical
characterization of fluorene and benzimidazole containing novel conjugated
polymers: Effect of alkyl chain length on electrochemical properties. European
Polymer Journal, 49(10):
3181-3187.
11. Nam,
S. W., Kang, S. H. and Chang, J. Y. (2007). Synthesis and photopolymerization
of photoreactive mesogens based on chalcone. Macromolecular
Research, 15(1):
74-81.
12. Tay,
M. G., Tiong, M. H., Chia, Y. Y., Kuan, S. H. C. and
Liu, Z. Q. (2016). A way to improve luminescent efficiency of bis-chalcone
derivatives. Journal of Chemistry, 2016: 3608137.
13. Kumar,
R., Kumar, A., Deval, V., Gupta, A., Tandon, P., Patil, P. S., Desmukh, P., Chaturvedi, D. and Watve,
J. G. (2017). Molecular structure, spectroscopic (FT-IR, FT Raman, UV, NMR and
THz) investigation and hyperpolarizability studies of
3-(2-Chloro-6-fluorophenyl)-1-(2-thienyl) prop-2-en-1-one. Journal of
Molecular Structure, 1129:
292-304.
14. Rahamathullah, R.
and Khairul, W. M. (2017). Evaluation on the electrochemically deposited alkoxy
thiourea as liquid crystalline semiconductor film. Applied Surface
Science, 424: 45-51.
15. Veeramanikandan, S., Sherine, H. B., Dhandapani, A. and Subashchandrabose,
S. (2019). Synthesis, solid state structure, Hirshfeld
surface, nonlinear optics and DFT studies on novel bischalcone
derivative. Journal of Molecular Structure, 1180: 798-811.
16. Castro,
G. T., Filippa, M. A., Sancho, M. I., Gasull, E. I. and Almandoz, M. C.
(2020) Solvent effect on the solubility and absorption spectra of meloxicam:
experimental and theoretical calculations. Physics and Chemistry of
Liquids, 58(3):
337-348.
17. Ustabaş, R., Süleymanoğlu, N., Özdemir,
N., Kahriman, N., Bektaş,
E. and Ünver, Y. (2020). New chalcone derivative:
Synthesis, characterization, computational studies and antioxidant activity. Letters
in Organic Chemistry, 17 (1):
46-53.
18. Shruthi,
C., Ravindrachary, V., Guruswamy, B., Prasad, D. J., Goveas, J., Kumara, K. and Lokanath,
N. K. (2021). Molecular structure, Hirshfeld surface
and density functional theoretical analysis of a NLO active chalcone derivative
single crystal-A quantum chemical approach. Journal of Molecular
Structure, 1228:
129739.
19. Kumar,
R., Kumar, A., Deval, V., Gupta, A., Tandon, P., Patil, P. S., Deshmukh, P.,
Chaturvedi, D. and Watve, J. G. (2017). Molecular
structure, spectroscopic (FT-IR, FT Raman, UV, NMR and THz) investigation and
hyperpolarizability studies of 3-(2-Chloro-6-fluorophenyl)-1-(2-thienyl)
prop-2-en-1-one. Journal of Molecular Structure, 1129: 292-304.
20. Naik,
K. M. and Nandibewoor, S. T. (2012). Electrochemical behavior of chalcone at a glassy carbon electrode and its
analytical applications. American Journal of Analytical Chemistry, 3(9): 656-663.
21. Baggio,
R., Brovelli, F., Moreno, Y., Pinto, M. and
Soto-Delgado, J. (2016). Structural, electrochemical and theoretical study of a
new chalcone derivative containing 3-thiophene rings. Journal of
Molecular Structure, 1123:
1-7.
22. Ilhan,
S., Temel, H., Yilmaz, I. and Sekerci,
M. (2007). Synthesis, structural characterization and electrochemical studies
of new macrocyclic Schiff base containing pyridine head and its metal complexes. Journal
of Organometallic Chemistry, 692
(18): 3855-3865.