Malaysian
Journal of Analytical Sciences Vol 26 No 4
(2022): 914 - 923
CHANGES OF FATTY ACID
COMPOSITION IN SCLERACTINIAN CORAL, Galaxea fascicularis (LINNAEUS,
1767) BY ACUTE EXPOSURE OF IRGAROL-1051
(Perubahan Komposisi Asid
Lemak dalam Karang Scleractinia, Galaxea fascicularis (LINNAEUS, 1976)
oleh Pededahan Akut Irgarol-1051)
Hassan Rashid Ali1, Che Din Mohd Safuan2,
Marinah Mohd Ariffin3, Mohammed Ali Sheikh1, Noor Azhar
Mohamed Shazili2, Aminudin Muhammad Afiq-Firdaus2,
Zainudin Bachok2*
1Tropical Research Centre for Oceanography, Environment and Natural
Resources,
The State University of Zanzibar, P. O. Box 146,
Zanzibar-Tanzania
2Institute of Oceanography and Environment
3Faculty of Science and Marine and Environment
University Malaysia Terengganu, 21030 Kuala Nerus,
Terengganu, Malaysia
*Corresponding
author: zainudinb@umt.edu.my
Received: 20 February 2022;
Accepted: 18 May 2022; Published: 25
August 2022
Abstract
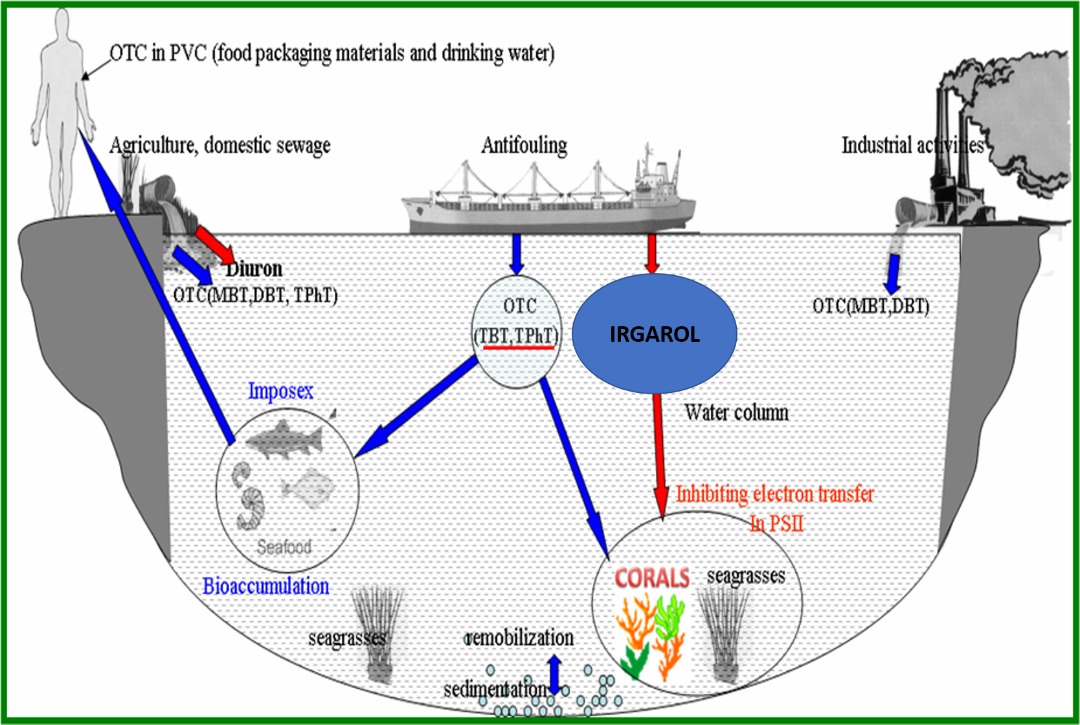
Antifouling biocide such as Irgarol 1051 has been widely used as a
replacement of tributyl tin (TBT). In Malaysia, we reported the level of
Irgarol in coastal water up to 2021ng/L. This raises concern because high
dosage of chemical pollutant in the seawater can affect the marine
organisms. This study therefore,
examined the effect of Irgarol 1051 on fatty acids composition of reef building
coral Galaxea fascicularis, collected in Pulau Bidong, Malaysia.
The corals were exposed to different doses of Irgarol 1051 under short
term exposure (96 hrs) and the fatty acid compositions of the coral tissues
were determined using the gas chromatography technique. The findings revealed no clear different (p >0.05)
among untreated samples (fresh and control) and both were dominated by
polyunsaturated fatty acids (PUFA), followed by saturated fatty acids (SAFA)
and monounsaturated fatty acids (MUFA). In contrast, the treated samples of G.
fascicularis (20, 100 and 500 µg/L) were significant different (p
<0.05) where both SAFA and PUFA were significantly lowered than untreated
samples especially at the samples exposed to higher dose of Irgarol 1051 (100
and 500 µg/L). As the level of dose increased, SAFA such as 16:0 and
unsaturated fatty acid from ω3 and ω6 series were largely affected by
the toxicology effect of the Irgarol 1051. The results indicate that Irgarol
1051 significantly affecting the health of the corals even at the lowest dose
of Irgarol 1051 applied in this study. It is suggested that the antifouling
biocide may have implication on metabolisms of the corals.
Keywords:
booster biocides, antifouling chemicals, fatty acids, hard coral, coral
reefs
Abstrak
Biosid anti-kotoran seperti Irgarol 1051 telah digunakan
secara meluas sebagai pengganti kepada tributil tin (TBT). Di Malaysia, kami
telah melaporkan tahap Irgarol di perairan pantai mencapai setinggi 2021ng/L.
Ini menimbulkan kebimbangan kerana dos bahan pencemar kimia yang tinggi dalam
air laut boleh menjejaskan organisma marin. Oleh itu, kajian ini mengkaji kesan
Irgarol 1051 pada komposisi asid lemak di dalam karang keras Galaxea fascicularis. Pendedahan jangka
pendek (96 jam) telah dilakukan ke atas spesis karang ini dengan menggunakan
kepekatan Irgarol yang berbeza dan komposisi asid lemak didalam tisu karang
ditentukan dengan menggunakan teknik gas kromatografi. Hasil kajian mendapati
tiada perubahan yang ketara pada karang yang tidak terdedah dengan Irgarol
(sampel segar dan kawalan) dan kedua-duanya mempunyai kandungan asid lemak yang
didominasi oleh asid lemak poli tidak tepu (PUFA), diikuti dengan asid lemak
tepu (SAFA) serta asid lemak mono tidak tepu (MUFA). Sebaliknya, terdapat
perbezaan yang ketara antara sampel karang yang tededah dengan kepekatan
berbeza Irgarol (20, 100 and 500 µg/L) dimana komposisi SAFA dan PUFA lebih
rendah berbanding sampel segar dan kawalan, terutamanya pada sampel yang
terdedah pada kepekatan Irgarol 1051 yang tinggi. SAFA seperti 16:0 dan asid
lemak tidak tepu dari kumpulan ω3 dan ω6 adalah antara asid lemak
yang sangat terkesan terhadap pendedahan pada bahan kimia ini. Dapatan kajian
juga menunjukkan, Irgarol 1051 sangat mempengaruhi kesihatan karang walaupun
hanya terdedah pada dos yang rendah. Ini menunjukkan bahawa terdapat implikasi
pada metabolisma karang apabila terdedah kepada bahan kimia ini.
Kata
kunci: biosid penggalak, bahan kimia
anti-kotoran, asid lemak, karang keras, terumbu karang
Graphical Abstract
References
1.
Malato, S.,
Blanco, J., Cáceres, J., Fernández-Alba, A. R., Agüera, A. and Rodríguez, A.
(2002). Photocatalytic treatment of water-soluble pesticides by photo-Fenton
and TiO2 using solar energy. Catalysis Today, 76(2-4):
209-220.
2.
Ali, H. R.,
Arifin, M. M., Sheikh, M. A., Mohamed Shazili, N. A. and Bachok, Z. (2013).
Occurrence and distribution of antifouling biocide Irgarol-1051 in coastal
waters of Peninsular Malaysia. Marine Pollution Bulletin, 70(1-2):
253-257.
3.
Harino, H.,
Arai, T., Ohji, M., Ismail, A. and Miyazaki, N. (2009). Contamination profiles
of antifouling biocides in selected coastal regions of Malaysia. Archives of
Environmental Contamination and Toxicology, 56(3): 468-478.
4.
Sheikh, M. A.,
Higuchi, T., Fujimura, H., Imo, T. S., Miyagi, T. and Oomori, T. (2009).
Contamination and impacts of new antifouling biocide Irgarol-1051 on
subtropical coral reef waters. International Journal of Environmental
Science and Technology, 6(3): 353-358.
5.
Bao, V. W. W.,
Leung, K. M. Y., Qiu, J. W. and Lam, M. H. W. (2011). Acute toxicities of five
commonly used antifouling booster biocides to selected subtropical and
cosmopolitan marine species. Marine Pollution Bulletin, 62(5):
1147-1151.
6.
West, K. and
Van Woesik, R. (2001). Spatial and temporal variance of river discharge on
Okinawa (Japan): Inferring the temporal impact on adjacent coral reefs. Marine Pollution Bulletin, 42(10):
864-872.
7.
Kitada, Y.,
Kawahata, H., Suzuki, A. and Oomori, T. (2008). Distribution of pesticides and
bisphenol a in sediments collected from rivers adjacent to coral reefs. Applied Catalysis B: Environmental,
82(3-4): 163-168.
8.
Omija, T.
(2004). Corals and Coral Reefs, In Coral Reefs of Japan. Ministry of
Environment and Japanese Coral Reef Society, Tokyo, pp. 64–68.
9.
Knutson, S.,
Downs, C. A. and Richmond, R. H. (2012). Concentrations of Irgarol in selected
marinas of Oahu, Hawaii and effects on settlement of coral larval. Ecotoxicology, 21(1): 1-8.
10.
Ali, H. R.,
Arifin, M. M., Sheikh, M. A., Mohamed Shazili, N. A. and Bachok, Z. (2015).
Toxicological studies of Irgarol-1051 and its effects on fatty acid composition
of Asian sea-bass, Lates calcarifer. Regional Studies in Marine Science, 2:
171-176.
11.
Cragg, B. A.
and Fry, J. C. (1984). The use of microcosms to simulate field experiments to
determine the effects of herbicides on aquatic bacteria. Journal General Microbiology, 130: 2309-2316.
12.
Sumpono
Perotti, P., Belan, A., Forestier, C., Lavedrine, B. and Bohatier, J. (2003).
Effect of diuron on aquatic bacteria in laboratory-scale wastewater treatment
ponds with special reference to Aeromonas species studied by colony
hybridization. Chemosphere, 50:
445-455.
13.
American Public
Health Association (1995). Standard method for the examination of water and
wastewater, nineteenth edition. Washington, DC.
14.
Abdulkadir, S.
and Tsuchiya, M. (2008). One-step method for quantitative and qualitative
analysis of fatty acids in marine animal samples. Journal of Experimental Marine Biology and Ecology, 354(1): 1-8.
15.
Bachok, Z.,
Arifin, M. M., Sheikh, M. A., Mohamed Shazili, N. A. and Ali, H. R. (2016).
Effects of Irgarol -1051 on fatty acid profile of solitary corals, Fungia fungites after acute exposure. Malaysian Journal of Analytical Sciences, 20(4):
697-703.
16.
Imbs, A. B.,
Demidkova, D. A., Latypov, Y. Y. and Pham, L. Q. (2007). Application of fatty
acids for chemotaxonomy of reef-building corals. Lipids, 42: 1035-1046.
17.
Imbs, A. B.,
Yakovleva, I. M., Latyshev, N. A. and Pham, L. Q. (2010). Biosynthesis of
polyunsaturated fatty acids in zooxanthellae and polyps of corals. Russian Journal of Marine Biology,
36(6): 452-457.
18.
Harland, A. D.,
Navarro, J. C., Spencer Davies, P. and Fixter, L.M. (1993). Lipids of some
Caribbean and Red Sea corals: total lipid, wax esters, triglycerides and fatty
acids. Marine Biology, 117: 113-117.
19.
Kumar, M.,
Kumari, P., Gupta, V., Anisha, P. A., Reddy, C. R. K. and Jha, B. (2010).
Differential responses to cadmium induced oxidative stress in marine macroalga Ulva
lactuca (Ulvales, Chlorophyta). Biometals
23: 315-325.
20.
Mohd Safuan, C.
D., Samshuri, M. A., Jaafar, S. N. T., Tan, H. C. and Bachok, Z. (2021).
Physiological response of shallow-water hard coral Acropora digitifera to heat stress via fatty acid composition. Frontiers in Marine Science, 2021: 1187.
21.
Filimonova, V.,
Goncalves, F., Marques, J. C., De Troch, M. and Goncalves, A. M. (2016). Fatty
acid profiling as bioindicator of chemical stress in marine organisms: a
review. Ecological Indicators, 67:
657-672.
22.
Regoli, F. and
Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative
stress biomarkers in marine organisms. Marine
Environmental Research, 93: 106-117.
23.
Downs, C. and
Downs, A. (2007). Preliminary examination of short-term cellular toxicological
responses of the coral Madracis mirabilis
to acute Irgarol 1051 exposure. Archives
of Environmental Contamination and Toxicology, 52(1): 47-57.
24.
Baird, A. H.,
Bhagooli, R., Ralph, P. J. and Takahashi, S. (2008). Coral bleaching: the role
of the host. Trends in Ecology &
Evolution, 24(1): 16-20.
25.
Rodríguez‐Troncoso, A. P., Carpizo‐Ituarte, E. and Cupul‐Magaña, A. L. (2016). Physiological response to high temperature in the
Tropical Eastern Pacific coral Pocillopora
verrucosa. Marine Ecology, 37(5):
1168-1175.
26.
Imbs, A. B. and
Yakovleva, I. M. (2012). Dynamics of lipid and fatty acid composition of
shallow-water corals under thermal stress: an experimental approach. Coral Reefs, 31(1): 41-53.
27.
Okuyama, H.,
Orikasa, Y. and Nishida, T. (2008). Significance of antioxidative functions of
eicosapentaenoic and docosahexaenoic acids in marine microorganisms. Applied and Environmental Microbiology,
74(3): 570-574.
28.
Papina, M.,
Meziane, T. and Van Woesik, R. (2003). Symbiotic zooxanthellae provide the
host-coral Montipora digitata with polyunsaturated fatty acids. Comparative Biochemistry and Physiology Part
B: Biochemistry and Molecular Biology, 135(3): 533-537.
29.
Jones, R. J.
and Kerswell, A. P. (2003). Phytotoxicity of Photosystem II (PSII) herbicides
to coral. Marine Ecology Progress Series,
261: 149-159.
30.
Teece, M. A.,
Estes, B., Gelsleichter, and E., Lirman, D. (2011). Heterotrophic and
autotrophic assimilation of fatty acids by two scleractinian corals, Montastraea faveolata and Porites astreoides. Limnology and Oceanography, 56(4): 1285-1296.
31.
Kamei, M.,
Takayama, K., Ishibashi, H., Takeuchi, I. (2020). Effects of ecologically
relevant concentrations of Irgarol 1051 in tropical to subtropical coastal
seawater on hermatypic coral Acropora
tenuis and its symbiotic dinoflagellates. Marine Pollution Bulletin, 150: 110734.