Malaysian
Journal of Analytical Sciences Vol 26 No 4
(2022): 698 - 707
EVALUATION OF THE TOTAL FLAVONOID, PHENOLIC CONTENT,
AND ANTIOXIDANT ACTIVITY IN SABAH SNAKE GRASS EXTRACTS (Clinacanthus nutans
Lindau)
(Penilaian Jumlah Kandungan Flavonoid,
Fenolik dan Aktiviti Antioksida bagi Ekstrak Belalai Gajah (Clinacanthus nutans Lindau))
Idham Zaharudie and Chong Shu Xian*
Faculty of Pharmacy,
SEGi University, No. 9, Jalan Teknologi, Taman Sains
Selangor, Kota Damansara, PJU 5,
47810 Petaling Jaya, Selangor, Malaysia
*Corresponding author: chongshuxian@segi.edu.my
Received: 3 March 2022; Accepted: 12 April 2022; Published: 25 August 2022
Abstract
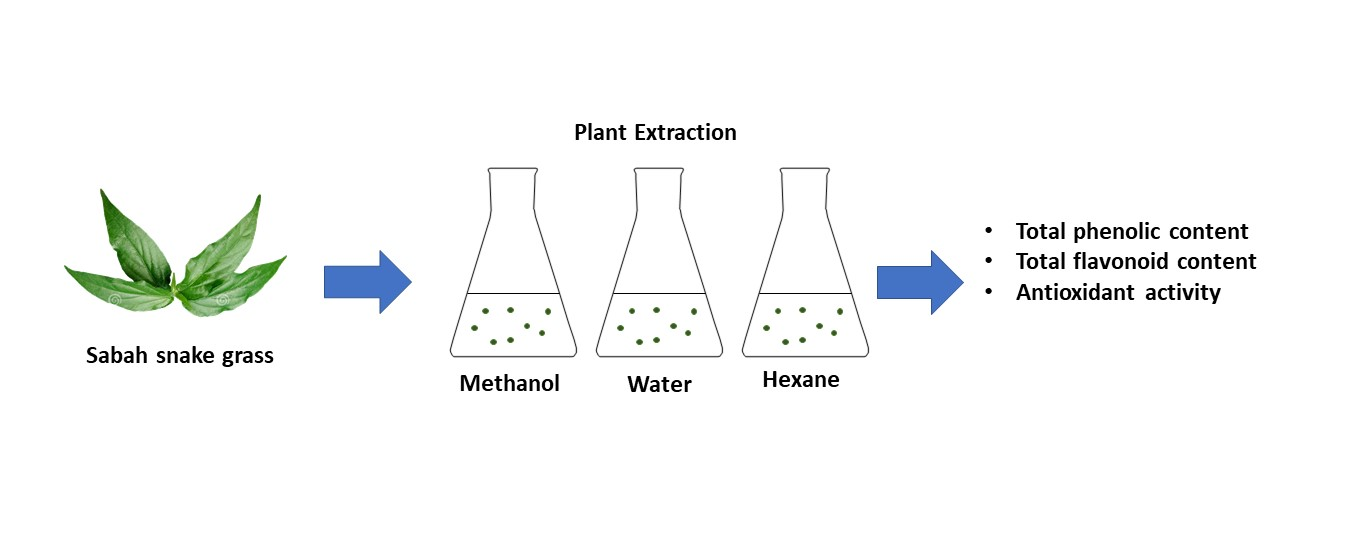
Clinacanthus nutans Lindau (C. nutans) or locally known in Malaysia as the Sabah Snake Grass (SSG)
contains phytochemicals such as flavonoids and phenolic acids. The leaves of
the plant exhibit antioxidant, anti-inflammatory, and antiviral properties,
thus, it was traditionally used to treat venomous injuries, rheumatism, and
sprains throughout Asia. Previous studies of the antioxidative activity in C.
nutans extracts have been carried out under different conditions or using
different extraction methods, which may have resulted in a varied yield of
antioxidative constituents. Thus, this study aimed to compare the total
phenolic content (TPC), total flavonoid content (TFC), and antioxidative
activity of C. nutans methanol, water, and hexane extracts using the
traditional solvent extraction method. Results showed that the water extract
contains the highest TPC value (3.66 ± 0.11 mg GAE/g) followed by methanol
(2.84 ± 0.36 mg GAE/g) and hexane (0.13 ± 0.03 mg GAE/g). The TPC values of
methanol and water extracts were significantly higher than hexane (p <
0.05). For TFC value, the methanol extract was the highest (1.91 ± 0.05 mg
QE/g), followed by water extract (1.50 ± 0.01 mg QE/g) and hexane was not
detected. The radical scavenging activity (RSA) decreased in the order of
methanol > water > hexane extract. In conclusion, the study suggests that
high phenolic and flavonoid contents in C. nutans extracts may be the
key factor for it to act as an excellent source of natural antioxidants.
Keywords: Sabah snake grass, total phenolic content, total flavonoid
content, radical scavenging activity
Abstrak
Clinacanthus
nutans Lindau (C. nutans) atau lebih dikenali oleh penduduk
tempatan di Malaysia sebagai belalai gajah mengandungi fitokimia seperti
flavonoid dan asid fenolik. Dedaun tumbuhan berikut mempamerkan sifat-sifat
seperti antioksida, anti radang, dan antivirus. Oleh itu, ia secara tradisionalnya
digunakan di seluruh asia untuk merawat kecederaan berbisa, sakit sendi, dan
seliuh. Kajian terdahulu mengenai aktiviti antioksida dalam ekstrak C.
nutans menggunakan kaedah ekstrak yang berbeza atau dalam keadaan yang
berbeza. Justeru, ini boleh menjana hasil kandungan antioksidan yang berbeza.
Kajian ini betujuan membuat penilaian jumlah kandungan fenolik (TPC), flavonoid
(TFC), dan aktiviti antioksida bagi ekstrak metanol, air, dan heksana daripada C.
nutans dengan kaedah pengekstrakan pelarut tradisional. Keputusan
menunjukkan bahawa ekstrak air mengandungi kandungi TPC tertinggi (3.66 ± 0.11
mg GAE/g) diikuti metanol (2.84 ± 0.36 mg GAE/g) and heksana (0.13 ± 0.03 mg
GAE/g). Bagi nilai TFC, ekstrak methanol adalah tertinggi (1.91 ± 0.05 mg QE/g),
mingikuti ekstrak air (1.50 ± 0.01 mg QE/g) dan heksana (tidak dikesan). Nilai
TPC ekstrak metanol dan air adalah jauh lebih tinggi daripada heksana (p <
0.05). Perencatan radikal bebas (RSA) bagi ekstrak dalam tertib menurun adalah
seperti berikut; metanol > heksana > air. Konklusinya, kajian ini
mencadangkan bahawa kandungan fenolik dan flavonoid yang tinggi dalam ekstrak C.
nutans mungkin menjadi faktor utama untuk ia bertindak sebagai sumber
antioksida semula jadi yang hebat.
Kata kunci: belalai
gajah, jumlah kandungan flavonoid, jumlah kandungan fenolik, perencatan radikal
bebas
Graphical Abstract
References
1.
Intan, S., Mahiran, B., Kim, W.,
Siti, E., Hamid, R. and Maznah, I. (2015). In vitro antioxidant, cytotoxic and
phytochemical studies of Clinacanthus nutans Lindau leaf extracts. African
Journal of Pharmacy and Pharmacology, 9(34): 861-874.
2.
Zulkipli, I., Rajabalaya, R., Idris,
A., Sulaiman, N. and David, S. (2017). Clinacanthus nutans: A review on
ethnomedicinal uses, chemical constituents and pharmacological properties. Pharmaceutical
Biology, 55(1): 1093-1113.
3.
Yahaya, R., Dash, G. K., Abdullah, M.
S. and Mathews, A. (2015). Clinacanthus nutans (burm. F.) Lindau: An useful
medicinal plant of south-east Asia, International Journal of Pharmacognosy
and Phytochemical Research, 7(6): 1244-1250.
4.
P'ng, X., Akowuah, G. and Chin, J.
(2013). Evaluation of the sub–acute oral toxic effect of methanol extract of Clinacanthus
nutans leaves in rats. Journal of Acute Disease, 2(1): 29-32.
5.
Siew, Y., Zareisedehizadeh, S.,
Seetoh, W., Neo, S., Tan, C. and Koh, H. (2014). Ethnobotanical survey of usage
of fresh medicinal plants in Singapore. Journal of Ethnopharmacology,
155(3): 1450-1466.
6.
Globinmed.com (2021). GlobinMed – a
unique interactive e-database with validated, up-to-date and comprehensive
information on integrated medicine. http://www.globinmed.com/ index.php?option=com_content&view=article&id=79320:clinacanthus-nutans-burmf-lindau&catid
=199:safety-of-herbal&Itemid=139 [Accessed online 22 August 2021].
7.
Pannangpetch, P., Laupattarakasem,
P., Kukongviriyapan, V., Kukongviriyapan, U., Kongyingyoes, B. and Aromdee, C.
(2007). Antioxidant activity and protective effect against oxidative hemolysis
of Clinacanthus nutans (Burm.f) Lindau. Songklanakarin Journal
of Science and Technology, 29: 1-9.
8.
Kong, H., Musa, K. and Abdullah Sani,
N. (2016). Clinacanthus nutans (Belalai Gajah/Sabah Snake Grass):
Antioxidant optimization on leaves and stems. AIP Conference Proceedings, 1784:
0300301-0300305.
9.
Jayavasu, C., Dechatiwongse, T. and
Balachandra, K. (1992). Virucidal activity of Clinacanthus nutans Lindau
extracts against herpes simplex virus type-2: In vitro study. Warasan Krom
Witthayasat Kanphaet, 34 (4): 153-158.
10.
Ghasemzadeh, A., Nasiri, A., Jaafar,
H., Baghdadi, A. and Ahmad, I. (2014) Changes in phytochemical synthesis, chalcone
synthase activity and pharmaceutical qualities of Sabah snake grass (Clinacanthus
nutans L.) in relation to plant age. Molecules, 19(11): 17632-17648.
11.
Halliwell, B. (1990) How to
characterize a biological antioxidant. Free Radical Research Communications,
9(1): 1-32.
12.
Krauss, R., Eckel, R., Howard, B.,
Appel, L., Daniels, S., Deckelbaum, R. (2000). The american heart association
dietary guidelines. Circulation, 102(18): 2284-2299.
13.
Yuann, J. M. P.,
Wang, J. S., Jian, H. L., Lin, C. C. and Liang, J. Y. (2012). Effects of Clinacanthus
nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA
from riboflavin photoreaction. MC-Transaction on Biotechnology, 4:
45-58.
14.
Sarega, N., Imam, M. U., Md Esa, N.,
Zawawi, N. and Ismail, M. (2016). Effects of phenolic-rich extracts of Clinacanthus
nutans on high fat and high cholesterol diet-induced insulin resistance. BMC
Complementary and Alternative Medicine,
16(1): 1-11.
15.
Yong, Y. K., Tan, J. J., The, S. S.,
Mah, S. H., Ee, G. C. L., Chiong, H. S. and Ahmad, Z. (2013). Clinacanthus
nutans extracts are antioxidant with antiproliferative effect on cultured
human cancer cell lines. Evidence-Based Complementary and Alternative Medicine,
2013: 462751.
16.
Khoo, L.W., Mediani, A., Zolkeflee,
N. K. Z., Leong, S. W., Ismail, I. S., Khatib, A., Shaari, K. and Abas, F.
(2015). Phytochemical diversity of Clinacanthus nutans extracts and
their bioactivity correlations elucidated by NMR based metabolomics. Phytochemistry
Letters, 14:123-133.
17.
Ismail, N. Z., Md Toha, Z., Muhamad,
M., Nik Mohamed Kamal, N. N. S., Mohamad Zain, N. N. and Arsad, H. (2020).
Antioxidant effects, antiproliferative effects, and molecular docking of Clinacanthus
nutans leaf extracts. Molecules, 25: 2067.
18.
Ghasemzadeh, A., Nasiri, A., Jaafar,
H. Z. E., Baghdadi, A. and Ahmad, I. (2014). Changes in phytochemical
synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah
snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules,
19: 17632-17648.
19.
Abd Samat, N. M.A., Ahmad, S., Awang,
Y., Bakar, R. A. H. and Hakiman, M. (2020). Alterations in herbage yield,
antioxidant activities, phytochemical contents, and bioactive compounds of
Sabah snake grass (Clinacanthus Nutans L.) with regards to harvesting
age and harvesting frequency. Molecules, 25 (12): 2833.
20.
Alam, M. A, Zaidul, I. S., Ghafoor,
K., Sahena, F., Hakim, M. A., Rafii, M.Y., Abir, H.M., Bostanudin, M. F.,
Perumal, V. and Khatib, A. (2017). In vitro antioxidant and, α-glucosidase
inhibitory activities and comprehensive metabolite profiling of methanol
extract and its fractions from Clinacanthus nutans. BMC Complementary
and Alternative Medicine, 17(1): 181.
21.
Haron, N. H., Md Toha, Z., Abas, R.,
Hamdan, M. R., Azman, N., Khairuddean, M. and Arsad, H. (2019). In vitro
cytotoxic activity of Clinacanthus nutans leaf extracts against HeLa
cells. Asian Pacific Journal of Cancer Prevention, 20(2): 601-609.
22.
Sarega, N., Imam, M. U., Ooi, D. J.,
Chan, K. W., Md Esa, N., Zawawi, N. and Ismail, M. (2016). Phenolic rich
extract from Clinacanthus nutans attenuates hyperlipidemia-associated
oxidative stress in rats. Oxidative Medicine and Cellular Longevity,
2016: 4137908.
23.
Ioannou, I., Chekir, L. and Ghoul, M.
(2020). Effect of heat treatment and light exposure on the antioxidant activity
of flavonoids. Processes, 8: 1078.
24.
Tushar, D., Sonal, S., Gajbhiye, N.
A. and Satyanshu, K. (2017). Effect of extraction methods on yield,
phytochemical constituents and antioxidant activity of Withania somnifera.
Arabian Journal of Chemistry, 10(1): S1193-S1199.
25.
Iqbal, E., Salim, K. and Lim, L.
(2015). Phytochemical screening, total phenolics and antioxidant activities of
bark and leaf extracts of Goniothalamus velutinus (airy shaw) from
Brunei Darussalam, Journal of King Saud University - Science, 27(3):
224-232.
26.
Rakholiya,
K., Kaneria, M., Nagani, K., Patel, A. and Chanda, S. (2015). Comparative
analysis and simultaneous quantification of antioxidant capacity of four
terminalia species using various photometric assays. World Journal of
Pharmaceutical Research, 4 (4): 1280-1296.
27.
Ismail, H., Chan, K., Mariod, A. and
Ismail, M. (2010). Phenolic content and antioxidant activity of
cantaloupe (Cucumis melo) methanolic extracts. Food Chemistry,
119(2): 643-647.
28.
Naczk, M. and Shahidi, F. (2006).
Phenolics in cereals, fruits and vegetables: Occurrence, extraction and
analysis. Journal of Pharmaceutical and Biomedical Analysis, 41(5):
1523-1542.
29.
Hemwimon, S., Pavasant, P. and
Shotipruk, A. (2007). Microwave-assisted extraction of antioxidative
anthraquinones from roots of Morinda citrifolia. Separation and
Purification Technology, 54(1): 44-50.
30.
King,
A. and Young, G. (1999). Characteristics and occurrence of phenolic
phytochemicals. Journal of the American Dietetic Association, 99(2):
213-218.