Malaysian
Journal of Analytical Sciences Vol 26 No 4
(2022): 884 - 901
POLYPHENOL-MEDIATED GREEN SYNTHESIS OF ZINC
OXIDE AND THEIR ANTIBACTERIAL PROPERTIES: A NOVEL SIZE-CONTROLLED APPROACH
(Sintesis
Hijau dengan Mediasi Polifenol dan Ciri Antibakteria Zink Oksida: Pendekatan
Kawalan Saiz Baharu)
Neo Zhi Zing1, Balkis A. Talip1*,
Soon Chin Fhong2, Ainun Rahmahwati Ainuddin3, Hatijah
Basri1
1Faculty
of Applied Sciences and Technology,
2Microelectronics
and Nanotechnology-Shamsuddin Research Centre, Faculty of Electrical and
Electronics Engineering
3Nano
Structure and Surface Modification (NANOSURF), Faculty of Mechanical
Engineering and Manufacturing
Universiti
Tun Hussein Onn Malaysia, 86400 Parit Raja, Batu Pahat, Johor, Malaysia
*Corresponding
author: balkis@uthm.edu.my
Received: 15 March 2022 ; Accepted: 18 May 2022 ;
Published: 25 August 2022
Abstract
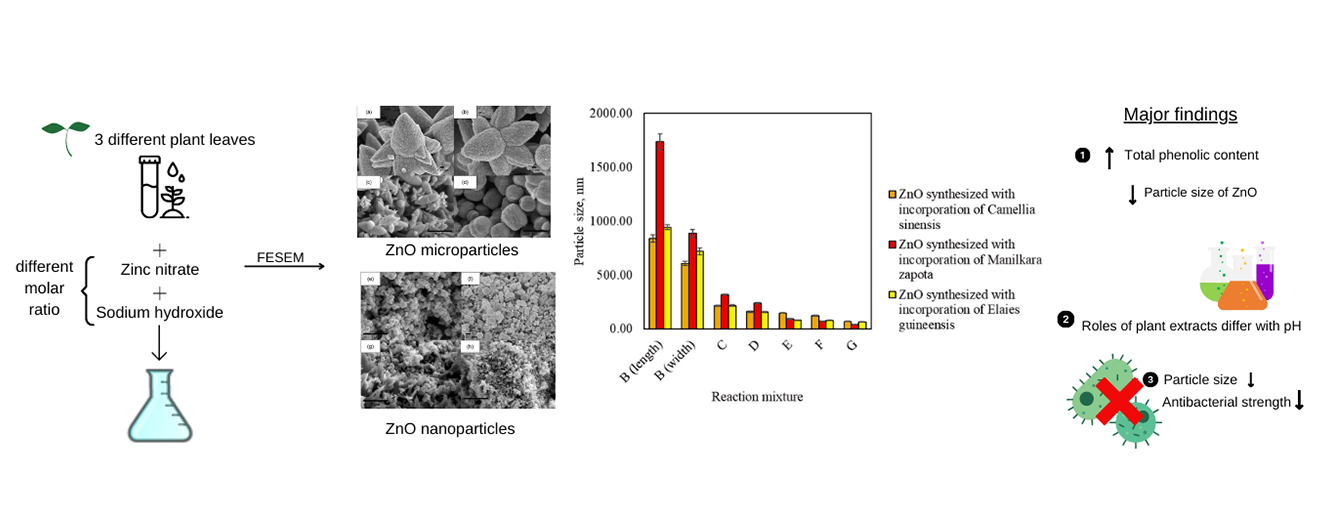
The concept of feasibility in green synthesis of zinc oxide (ZnO)
nanoparticles has been discussed in many studies. However, size control using
volume of plant extracts undermines the process upscaling potential. This study
is aimed at improving the repeatability and reproducibility of green synthesis
of ZnO by controlling total phenolic content of plant extracts. Leaf extracts
of Camellia sinensis, Manilkara zapota and Elaeis guineensis were incorporated at gallic acid equivalent of 100 mgg-1 to
synthesize ZnO. The phytochemical profile
of plant extracts and physical properties of ZnO were determined. In addition,
antibacterial activity of ZnO against Escherichia
coli and Staphylococcus aureus
was examined. Consistency in particle sizes of ZnO has
justified the feasibility of using total phenolic content for size control.
Under neutral pH, role of phytochemicals as chelating agents predominated.
Under basic condition, complex phytochemicals demonstrated structure-directing
effect on ZnO microparticles. The antibacterial
strength of ZnO has been reduced by 16 times with reduction in particle size.
Meanwhile, the incorporation of phytochemicals enhanced antibacterial activity
of ZnO by fourfold. This study proposed that particle size and morphology of ZnO could be
controlled through manipulation of total phenolic content of plant extracts and
the reaction pH of green synthesis.
Keywords: green synthesis, polyphenol, zinc oxide, size control
Abstrak
Konsep dalam kebolehlaksanaan proses sintesis hijau
terhadap nanopartikel zink oksida (ZnO) telah diperjelaskan dalam pelbagai
kajian. Namun begitu, kawalan saiz menggunakan isipadu ekstrak tumbuhan
menjejaskan potensi dalam meningkatkan skala proses. Kajian ini bertujuan untuk
meningkatkan kebolehulangan sintesis hijau ZnO dengan mengawal jumlah kandungan
fenolik ekstrak tumbuhan yang digabungkan. Ekstrak daun Camellia sinensis, Manilkara
zapota dan Elaeis guineensis
telah dicampurkan pada persamaan asid gallik sebanyak 100 mgg-1
untuk menghasilkan ZnO. Profil fitokimia ekstrak tumbuhan dan sifat fizikal ZnO
telah ditentukan. Di samping itu, aktiviti antibakteria ZnO terhadap Escherichia coli dan Staphylococcus aureus telah diperiksa.
Ketekalan dalam saiz partikel ZnO menjustifikasikan kebolehlaksaan penggunaan
jumlah kandungan fenolik untuk kawalan saiz dalam sintesis hijau. Di bawah pH
neutral, peranan fitokimia sebagai agen pengkelat berdominasi. Di bawah keadaan
alkali, fitokimia kompleks menunjukkan kesan pengarahan struktur pada
mikropartikel ZnO. Kekuatan antibakteria ZnO berkurangan sebanyak 16 kali ganda
dengan pengurangan saiz partikel. Sementara itu, penggunaan fitokimia dalam
proses sintesis telah meningkatkan aktiviti antibakteria ZnO sebanyak 4 kali
ganda. Kajian ini mencadangkan bahawa saiz partikel dan morfologi ZnO boleh
dikawal melalui manipulasi jumlah kandungan fenolik ekstrak tumbuhan dan pH
tindak balas sintesis hijau.
Kata kunci: sintesis
hijau, polifenol, zink oksida, kawalan saiz
Graphical Abstract
References
1.
Inshakova, E. and
Inshakov, O. (2017). World market for nanomaterials: structure and trends. MATEC Web of Conferences, 129: 02013.
2.
Jeevanandam, J.,
Barhoum, A., Chan, Y. S., Dufresne, A. and Danquah, M. K. (2018). Review on
nanoparticles and nanostructured materials: history, sources, toxicity and
regulations. Beilstein Journal of
Nanotechnology, 9: 1050-1074.
3.
Singh, J., Dutta,
T., Kim, K., Rawat, M., Samddar, P. and Kumar, P. (2018). ‘Green’ synthesis of
metals and their oxide nanoparticles: applications for environmental
remediation. Journal of
Nanobiotechnology, 16: 84.
4.
Zikalala, N.,
Matshetshe, K., Parani, S. and Oluwafemi, O. S. (2018). Biosynthesis protocols
for colloidal metal oxide nanoparticles. Nano-Struct.
Nano-Objects, 16: 288-299.
5.
Kharissova, O. V.,
Kharisov, B. I., González, C. M. O., Méndez, Y. P. and López, I. (2019).
Greener synthesis of chemical compounds and materials. Royal Society Open Sciences, 6: 191378.
6.
Rodríguez-León,
E., Rodríguez-Vázquez, B. E., Martínez-Higuera, A., Rodríguez-Beas, C.,
Larios-Rodríguez, E., Navarro, R. E., López-Esparza, R. and Ińiguez-Palomares,
R. A. (2019). Synthesis of gold nanoparticles using Mimosa tenuiflora extract, assessments of cytotoxicity, cellular
uptake, and catalysis. Nanoscale Research
Letters, 14: 334.
7.
Pal, S., Pal, K.,
Mukherjee, S., Bera, D., Karmakar, P. and Sukhen, D. (2020). Green cardamom
mediated phytosynthesis of ZnO NPs and validation of its antibacterial and
anticancerous potential. Materials
Research Express, 7: 015068.
8.
Soni, V., Raizada,
P., Singh, P., Cuong, H. N., Rangabhashiyam, S., Saini, A., Saini, R. V., Le,
Q. V., Nadda, A. K., Le, T. and Nguyen, V. (2021). Sustainable and green trends
in using plant extracts for the synthesis of biogenic metal nanoparticles
toward environmental and pharmaceutical advances: a review. Environmental Research, 202: 111622.
9.
Siddiqi, K. S., Ur
Rahman, A., Tajuddin and Husen, A. (2018). Properties of zinc oxide
nanoparticles and their activity against microbes. Nanoscale Research Letters, 13(1): 141.
10.
Pasquet, J.,
Chevalier, Y., Pelletier, J., Couval, E., Bouvier, D. A and Bolzinger, M.
(2014). The contribution of zinc
ions to the antimicrobial activity of zinc oxide. Colloids Surface A Physicochemical Engineering Aspects, 457:
263-274.
11.
Król, A.,
Pomastowski, P., Rafińska, K., Railean-Plugaru, V. and Buszewski, B.
(2017). Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity
mechanism. Advance Colloid Interface
Sciences, 249: 37-52.
12.
Xu, J., Huang, Y.,
Zhu, S., Abbes, N., Jing, X. and Zhang, L. (2021). A review of the green
synthesis of ZnO nanoparticles using plant extracts and their prospects for
application in antibacterial textiles. Journal
Engineering Fibers Fabrication, 16: 1-14.
13.
Câmara, J. S., Albuquerque, B. R., Aguiar, J.,
Corręa, R. C. G., Gonçalves, J. L., Granato, D., Pereira, J. A. M., Barros, L.
and Ferreira, I. C. F. R. (2020). Food bioactive compounds and emerging
techniques for their extraction: polyphenols as a case study. Foods (Basel, Switzerland), 10(1): 37.
14.
Belščak-Cvitanović,
A., Valinger, D., Benković, M, Tušek, A. J., Jurina, T., Komes, D. and
Kljusurić, J. G. (2017). Integrated approach for bioactive quality
evaluation of medicinal plant extracts using HPLC-DAD, spectrophotometric, near
infrared spectroscopy and chemometric techniques. International Journal Food Production, 20(3): 1-18.
15.
Almini, S. M. and
Akbari, A. (2019). Metal nanoparticles synthesis through natural phenolic
acids. IET Nanobiotechnology, 13(8):
771-777.
16.
Mercado-Mercado,
G., Blancas-Benitez, F. J., Velderrain-Rodríguez, G. R., Montalvo-González, E.,
González-Aguilar, G. A., Alvarez-Parrilla, E. and Sáyago-Ayerdi, S. G. (2015).
Bioaccessibility of polyphenols released and associated to dietary fibre in
calyces and decoction residues of Roselle (Hibiscus
sabdariffa L.). Journal Functional
Foods, 18: 171-181.
17.
Dudonné, S., Vitrac,
X., Coutiѐre, P., Woillez, M. and Mérillon, J. (2009). Comparative study of antioxidant properties and total phenolic content
of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and
ORAC assays. Journal Agriculture Food
Chemistry, 57: 1768-1774.
18.
Iqbal, E., Salim,
K. A. and Lim, L. B. L. (2015). Phytochemical screening, total phenolics and
antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. Journal King Saud University Sciences,
27: 224-232.
19.
Gul, R., Jan, S.
U., Faridullah, S., Sherani, S. and Jahan, N. (2017). Preliminary phytochemical
screening, quantitative analysis of alkaloids, and antioxidant activity of
crude plant extracts from Ephedra
intermedia indigenous to Balochistan. Science
World Journal, 2017: 5873648.
20.
Aiyegoro, O. A.
and Okoh, A. I. (2010). Preliminary phytochemical screening and In vitro antioxidant activities of the
aqueous extract of Helichrysum
longifolium DC. BMC Complementary
Alternative Medicine, 10: 21.
21.
Ukoha, P. O.,
Cemaluk, E. A. C., Nnamdi, O. L. and Madus, E. P. (2011). Tannins and other
phytochemicals of the Samanaea saman
pods and their antimicrobial activities. African
Journal Pure Applied Chemstry, 5(8): 237-244.
22.
El Aziz, M. M. A.,
Ashour, A. S. and Melad, A. S. G. (2019). A review on saponins from medicinal
plants: chemistry, isolation, and determination. Journal Nanomedicine Research, 7(4): 282-288.
23.
Shukla, S., Mehta,
A. and Bajpai, V. K. (2013). Phytochemical screening and anthelmintic and
antifungal activities of leaf extracts of Stevia
rebaudiana. Journal of Biologically Active Products from Nature,
3(1): 56-63.