Malaysian
Journal of Analytical Sciences Vol 26 No 4
(2022): 867 - 883
A BRIEF REVIEW ON CORROSION INHIBITION STUDY
OF ORGANIC LIGAND: ELECTROCHEMICAL, MORPHOLOGY, AND ISOTHERM STUDIES
(Ulasan
Ringkas Terhadap Kajian Perencatan Kakisan Ligan oleh Ligan Organik: Kajian
Elektrokimia, Morfologi dan Isoterma)
Nur
Nadia Dzulkifli1,3*, Nur Zalin Khaleda Razali3, Norsanida
Iswa Sahani3, Sheikh Ahmad Izaddin Sheikh Mohd Ghazali1,3,
Dzeelfa Zainal Abidin2, Asiah Abdullah1,3, Nurazira Mohd
Nor1,3
1Material, Inorganic, and
Oleochemistry (MaterInOleo) Research Group, Faculty of Applied Sciences
2Academy of Language Studies
3School of Chemistry and
Environment, Faculty of Applied Sciences
Universiti Teknologi MARA
Cawangan Negeri Sembilan, Kampus Kuala Pilah, Pekan Parit Tinggi,
72000 Kuala Pilah, Negeri
Sembilan, Malaysia
*Corresponding author:
nurnadia@uitm.edu.my
Received: 10 February 2022; Accepted: 2 April 2022;
Published: 25 August 2022
Abstract
Over the past decade, the corrosion inhibition of organic
ligands has been extensively studied in numerous experiments in acid media. The
number of published papers related to corrosion inhibition studies of organic
ligands has been rising exponentially. The organic ligands have high inhibitive
properties due to their capability to adsorb on the surface of metal by forming
a protective layer. Having lone pair electrons (S, N, O) and multiple bonds
(π bonds) allow them to adsorb on the surface of metals efficiently.
However, there is very limited and less comprehensive information on the
characterization of corrosion inhibition performance of organic ligands on the
surface of metals. Therefore, this review paper provides a comprehensive review
on the corrosion inhibition performance through various characterization
methods, which are the electrochemical method [Electrochemical Impedance
Spectroscopy (EIS), Polarization], Scanning Electron Microscope (SEM) with
Energy Dispersive X-ray (EDX), and Langmuir Isotherm, which are thoroughly
discussed herein.
Keywords: electrochemical impedance spectroscopy, polarization, scanning electron microscope with energy dispersive X-ray; Langmuir isotherm
Abstrak
Sepanjang
dekad yang lalu, perencatan kakisan ligan organik di dalam media berasid telah
diuji di dalam eksperimen secara meluas dalam banyak eksperimen dalam media
asid. Bilangan makalah yang diterbitkan berkaitan dengan kajian perencatan
kakisan ligan organik telah meningkat secara eksponen. Ligan organik mempunyai
sifat perencat yang tinggi kerana keupayaannya untuk menjerap pada permukaan
logam dengan membentuk lapisan pelindung. Mempunyai pasangan elektron tunggal
(S, N, O) dan ikatan berganda (ikatan π) membolehkan ligan organik
menjerap pada permukaan logam dengan berkesan. Walau bagaimanapun, terdapat maklumat
yang sangat terhad dan kurang komprehensif mengenai pencirian prestasi
perencatan kakisan ligan organik pada permukaan logam. Oleh itu, kertas kajian
ini menyediakan ulasan kajian secara menyeluruh tentang pencirian prestasi
perencatan kakisan melalui pelbagai kaedah pencirian seperti kaedah
elektrokimia [Spectroskopi Impedan Elektrokimia (EIS), Polarisasi], Mikroskop
Elektron Pengimbas (SEM) dengan Sinar-X Serakan Tenaga (EDX), dan Isoterma
Langmuir dan telah dibincangkan dengan teliti di sini.
Kata kunci: spektroskopi impedan elektrokimia, polarisasi, mikroskop elektron
pengimbas dengan sinar-X serakan tenaga, isoterma Langmuir
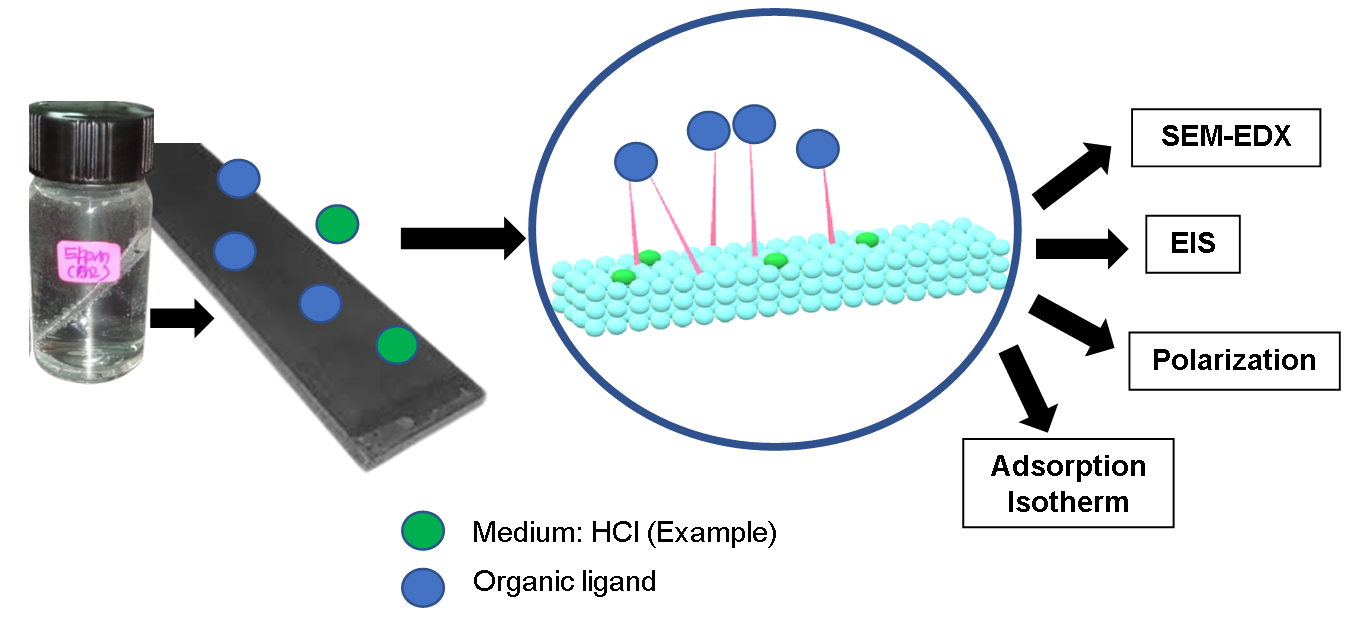
Graphical Abstract
References
1.
Damborenea, J. de.,
Conde, A. and Arenas, M. A.
(2014). Chapter 3: 3 - Corrosion inhibition with rare earth metal
compounds in aqueous solutions. Rare Earth-Based Corrosion Inhibitors. Woodhead Publishing Series in Metals and
Surface Engineering. Elsevier.
2.
Amitha, R. B. E. and Basu, B. B.
J. (2012). Green inhibitors for corrosion protection of metals and alloys: an
overview. International Journal of Corrosion,
2012: 1-15.
3.
Kumar, H. and Yadav, V. (2021). Highly efficient and eco-friendly acid
corrosion inhibitor for mild steel: Experimental and theoretical study. Journal of Molecular Liquid, 335: 1-16.
4. Danaee,
I., Bahramipanah, N., Moradi, S. and Nikmanesh, S. (2016). Impedance
spectroscopy studies on corrosion inhibition behavior of synthesized n,n’-bis(2,4-dihydroxyhydroxybenzaldehyde)-1,3-propandiimine
for API-5L-X65 steel in HCl solution. Journal
of Electrochemical Science and Technology, 7(2): 153-160.
5.
Thoume, A., Elmakssoudi, A., Benmessaoud, L. D., Benzbiria, N.,
Benhiba, F., Dakir, M., Zahouily, M., Zarrouk, A., Azzi, M. and Zertoubi, M.
(2020). Amino acid structure analog as a corrosion inhibitor of carbon steel in
0.5 M H2SO4: Electrochemical, synergistic effect and
theoretical studies. Chemical Data
Collections, 30: 1-18.
6.
Dehghani, A., Mostafatabar, A. H., Bahlakeh, G., Ramezanzadeh, B. and
Ramezanzadeh, M. (2020). Detailed-level computer modeling explorations
complemented with comprehensive experimental studies of Quercetin as a highly
effective inhibitor for acid-induced steel corrosion. Journal of Molecular Liquid, 309: 1-51.
7.
Beytur, M., Irak, Z. T., Manap, S. and Yuksek, H. (2019). Synthesis,
characterization and theoretical determination of corrosion inhibitor
activities of some new 4,5-dihydro-1H-1,2,4-Triazol-5-one derivatives. Heliyon, 5: 1-8.
8.
Ozkir,
D. (2019). A newly synthesized schiff base derived from
condensation reaction of 2,5-dichloroaniline and benzaldehyde: Its
applicability through molecular interaction on mild steel as an acidic
corrosion inhibitor by using electrochemical techniques. Journal
of Electrochemical Science and Technology, 10(1): 37-54.
9.
Marinescu, M. (2019). Recent advances
in the use of benzimidazoles as corrosion inhibitors. BMC
Chemistry, 13(136):
1-21.
10.
Jamil, D. M., Al-Okbi, A. K., Al-Baghdadi, S. B., Al-Amiery, A. A., Kadhim, A., Gaaz,
T. S., Kadhum, A. A. H. and Mohamad, A. B. (2018). Experimental and theoretical
studies of Schiff bases as corrosion inhibitors. Chemistry Central Journal, 12(7): 1-9.
11.
Padash, R., Rahimi-Nasrabadi, M., Rad, A.
S., Sobhani-Nasab, A., Jesionowski, T. and Ehrlich, H. (2019). A theoretical study of two novel Schiff
bases as inhibitors of carbon steel corrosion in acidic medium. Applied Physic A, 125(78): 1-11.
12.
Keles, H.¸ Emir, D. M. and Keles, M. (2015). A comparative study of
the corrosion inhibition of low carbon steel in HCl solution by an imine
compound and its cobalt complex. Corrosion
Science, 101: 19-31.
13.
Lgaz, H., Salghi, R., Jodeh, S. and Hammout, B. (2017). Effect of
clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. Journal of Molecular Liquid, 225:
271-280.
14. Khaled, K. F.,
Samardzija, K. B. and Hackerman, N. (2006). Cobalt(III) complexes of
macrocyclic-bidentate type as a new group of corrosion inhibitors for iron in
perchloric acid. Corrosion Science,
48: 3014-3034.
15.
Abdallah, M., Gad, E., Sobhi, M.,
Al-Fahemi, J. H. and Alfakeer, M. (2019). Performance of tramadol drug as a
safe inhibitor for aluminum corrosion in 1.0M HCl solution and understanding
mechanism of inhibition using DFT. Egyptian
Journal of Petroleum, 28(2): 173-181.
16.
Boughoues, Y., Benamira,
M., Messaadia, L., Bouider, N. and Abdelaziz, S. (2020). Experimental and theoretical
investigations of four amine derivatives as effective corrosion inhibitors for
mild steel in HCl medium. RSC Advances, 10: 24145-24158.
17.
Ferreira, E. S., Giancomelli,
C., Giacomelli,
F. C. and Spinelli, A. (2004). Evaluation of the inhibitor effect
of L-ascorbic acid on the corrosion of mild steel. Materials Chemistry and Physics, 83(1): 129-134.
18.
Nikoo, S. Z., Shockravi, A.,
Ghartavol, H. M., Halimehjani, A. Z., Ostadrahimi, M., Mirhosseini, S. M.,
Behzadi, H. and Ghorbani, M. (2020). A study of Glycine-based dithiocarbamates
as effective corrosion inhibitors for cold rolled carbon steel in HCl solutions.
Surfaces and Interfaces, 21: 1-67.
19.
Xu, B., Yang, W., Liu, Y., Yin,
X., Gong, W. and Chen, Y. (2014). Experimental and theoretical evaluation of
two pyridine carboxaldehyde thiosemicarbazone compounds as corrosion inhibitors
for mild steel in hydrochloric acid solution. Corrosion Science, 78: 260-268.
20.
Mahgoub, F. M., Abdel-Nabey,
B. A. and El-Samadisy, Y. A.
(2010). Adopting a
multipurpose inhibitor to control corrosion of ferrous alloys in cooling water
systems. Materials Chemistry
and Physics, 120(1): 104-108.
21. Geoffrey, B., Dang, D. N., Stephanie, M. and Sebastien, T. (2014). Analysis of the non-ideal capacitive behaviour for high
impedance organic coatings. Progress in
Organic Coatings, 77(12): 2045-2053.
22.
Aouniti, A., Elmsellema, H., Tighadouini, S., Elazzouzi, M., Radi, S.,
Chetouani, A., Hammouti, B. and Zarrouk, A. (2016). Schiff’s base derived from
2-acetyl thiophene as corrosion inhibitor of steel in acidic medium. Journal of Taibah University for Science,
10: 774-785.
23.
Prajila, M., Ammal, P. R. and Abraham, J. (2018). Comparative studies on the corrosion inhibition
characteristics of three different triazine based Schiff’s bases, HMMT, DHMMT
and MHMMT. Egyptian Journal of Petroleum,
27(4): 467-475.
24.
Chetouani, A., Medjahed, K.,
Benabadji, K. E., Hammouti,
B., Kertit, S. and Mansri, A.
(2003). Poly(4-vinylpyridine
isopentyl bromide) as inhibitor for corrosion of pure iron in molar sulphuric
acid. Progress in Organic Coatings,
46(4): 312-316.
25.
Aby, P., Joby, T. K., Vinod, P.
R. and Shaju, K. S. (2012). 3-Formylindole-4-aminobenzoic Acid: A potential
corrosion inhibitor for mild steel and copper in hydrochloric acid media. ISRN Corrosion, 2012: 1-10.
26.
Okonkwo, P. C., Sliem, M. H., Shakoor, R. A., Mohamed,
A. M. A. and Abdullah, A. M. (2017). Effect of temperature on the corrosion
behavior of API X120 pipeline steel in H2S environment. Journal of Materials Engineering and
Performance, 26: 3775-3783.
27.
Aytac, A., Ozmen, U. and Kabasakaloglu, A. (2005). Investigation
of some Schiff bases as acidic corrosion of alloy AA3102. Materials Chemistry and Physics, 89(1): 176-181.
28.
Mourya, P., Banerjee, S., Rastogi, R. B. and Singh, M. M. (2013).
Inhibition of mild steel corrosion in hydrochloric and sulfuric acid media
using a thiosemicarbazone derivative. Industrial & Engineering Chemistry Research, 52(36):
12733-12747.
29.
Elias, E. E., Henry, U. N. and Damian, C. O. (2018).
Synthesis and characterization of Schiff bases NBBA, MNBA and CNBA. Heliyon, 4(7): 1-25
30.
Jiyaul, H., Ansari, K. R.,
Vandana, S., Quraishi, M. A. and Obot, I. B. (2017). Pyrimidine derivatives as
novel acidizing corrosion inhibitors for N80 steel useful for petroleum
industry: A combined experimental and theoretical approach. Journal of Industrial and Engineering
Chemistry, 49: 176-188.
31.
Solmaz, R., Kardas, G., Çulha, M., Yazici, B. and Erbil, M. (2008). Investigation of adsorption and inhibitive effect of
2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media. Electrochimica
Acta, 53(20): 5941-5952.
32.
Khaled, K. F. (2010). Electrochemical behavior of nickel in nitric
acid and its corrosion inhibition using some thiosemicarbazone derivatives. Electrochimica Acta, 55: 5375-5383.
33.
Bhawna, C., Ashish, K. S., Sanjeeve, T., Balaram, P., Hassane, L.,
Ill-Min, C., Ranjana, J. and Eno, E. E. (2020). Comparative investigation of
corrosion-mitigating behavior of thiadiazole-derived bis-schiff bases for mild
steel in acid medium: experimental, theoretical, and surface study. ACS Omega, 5: 13503-13520.
34. El Basiony, N. M., Amr,
E., Nady, H., Migahed, M.
A. and Zaki, E. G. (2019). Adsorption
characteristics and inhibition effect of two Schiff base compounds on corrosion
of mild steel in 0.5 M HCl solution: experimental, DFT studies, and Monte
Carlo simulation. RSC Advances, 9: 10473-10485.
35.
Jacob, K. S. and Geetha, P. (2010). Corrosion inhibition of mild steel
in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corrosion Science, 52: 224-228.
36.
Idouhli,
R., Ousidi, A. N., Koumya, Y., Abouelfida, A., Benyaich, A., Auhmani, A. and
Moulay, Y. A. I. (2018). Electrochemical studies of monoterpenic
thiosemicarbazones as corrosion inhibitor for steel in 1 M HCl. International Journal of Corrosion,
2018: 1-15.
37.
Manilal, M., Sourav, K. S., Prabhas, B., Naresh, C. M., Harish, H. and
Priyabrata, B. (2020). Corrosion inhibition property of azomethine
functionalized triazole derivatives in 1 molL−1 HCl medium for
mild steel: Experimental and theoretical exploration. Journal of Molecular Liquid, 313: 1-15.
38.
Muthukrishnan, P., Prakash, P., Jeyaprabha, B. and Shankar, K. (2019).
Stigmasterol extracted from Ficus hispida
leaves as a green inhibitor for the mild steel corrosion in 1M HCl solution. Arabian Journal of Chemistry, 12(8):
3345-3356.
39.
Ammal, P. R., Prajila, M. and
Abraham, J. (2018). Physicochemical studies on the inhibitive properties of a
1,2,4-triazole Schiff’s base, HMATD, on the corrosion of mild steel in
hydrochloric acid. Egyptian Journal of
Petroleum, 27: 307-317.
40.
Chitra, S., Parameswari, K. and
Selvaraj, A. (2010). Dianiline Schiff bases as inhibitors of mild steel
corrosion in acid media. International
Journal of Electrochemical Science, 5: 1675-1697.
41.
Ilhem, K., Tahar, D., Djamel, D., Saifi, I., Lakhdar, S. and Salah, C.
(2021). Synthesis, characterization and anti-corrosion properties of two new
Schiff bases derived from diamino diphenyl ether on carbon steel X48 in 1M HCl.
Journal of Adhesion Science and
Technology, 35(6): 1-31.
42.
Shirin, S., Sarmin, H., Jahan, B. G., Parviz, N. and Alireza, S.
(2019). Synthesis, experimental, quantum chemical and molecular dynamics study
of carbon steel corrosion inhibition effect of two Schiff bases in HCl
solution. Journal of Molecular Liquid,
285: 626-639.
43.
Deng, X. and Li, X. X. (2014). Hydroxymethyl urea and
1,3-bis(hydroxymethyl) urea as corrosion inhibitors for steel in HCl solution. Corrosion Science, 80: 276-289.
44.
Uzma, N., Zareen, A., Naveed,
Z. A. and Faiz, U. S. (2019). Experimental and theoretical insights into the corrosion
inhibition activity of novel Schiff bases for aluminum alloy in acidic medium. RSC Advances, 9: 36455-36470.
45.
Khadraoui, A., Khelifa, M. H., Razika, M., Kamel, H., Tidu, A., Azari,
Z., Ime, B. O. and Zarrouk, A. (2016). Extraction, characterization and
anti-corrosion activity of Mentha
pulegium oil: Weight loss, electrochemical, thermodynamic and surface
studies. Journal of Molecular Liquid,
216: 724-731.
46.
Nimmy, K., Joby, T. K., Vinod, P.
R. and Shaju, K. S. (2014). Electrochemical impedance spectroscopy and
potentiodynamic polarization analysis on anticorrosive activity of
thiophene-2-carbaldehyde derivative in acid medium. Indian Journal of Materials Science, 2014: 1-6.
47.
Hamdani, N. E., Fdil, R., Tourabi, M., Jama, C. and Bentiss, F.
(2015). Alkaloids extract of Retama
monosperma (L.) Boiss. seeds used as novel eco-friendly inhibitor for
carbon steel corrosion in 1 M HCl solution: Electrochemical and surface
studies. Applied Surface Science,
357: 1294-1305.
48.
Turuvekere, K. C., Kikkeri, N. S.
M. and Harmesh, C. T. (2015). Thermodynamic, electrochemical and quantum
chemical evaluation of some triazole Schiff bases as mild steel corrosion
inhibitors in acid media. Journal of
Molecular Liquid, 211: 1026-1038.
49.
Ragi, K., Joby, T. K., Vinod, P.
R., Sini, V. C. and Binsi, M. P. (2019). Synthesis, cyclic voltammetric,
electrochemical, and gravimetric corrosion inhibition investigations of schiff
base derived from 5,5-dimethyl-1,3-cyclohexanedione and 2-aminophenol on mild
steel in 1 M HCl and 0.5 M H2SO4. International Journal of Electrochemistry, 2019: 1-13.
50.
Weihua,
L., Qiao,
H., Changling,
P. and Baorong,
H. (2007). Experimental and theoretical investigation
of the adsorption behaviour of new triazole derivatives as inhibitors for mild
steel corrosion in acid media. Electrochimica Acta, 52(22): 6386-6394.
51. Ifzan, A., Aamer,
S., Pervaiz, A. C., Syeda, A. S., Muhammad, N. A. and Muhammad, S. (2020).
Bis-Schiff bases of
2,2′-dibromobenzidine as efficient corrosion inhibitors for mild steel in
acidic medium. RSC Advances,
10: 4499-4511.
52.
Yadav, M., Kumar, S., Sinha, R. R. and Kumar, S. (2014). Experimental
and theoretical studies on synthesized compounds as corrosion inhibitor for
mild steel in hydrochloric acid solution. Journal
of Dispersion Science and Technology, 35: 1751-1763.
53.
Poorqasemi, E., Abootalebi, O., Peikari, M. and Haqdar, F. (2009).
Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corrosion Science, 51: 1043-1054.
54.
Sam, J., Jeevana, R., Aravindakshan, K. K. and Abraham, J. (2017).
Corrosion inhibition of mild steel by n(4)-substituted
thiosemicarbazone in hydrochloric acid media. Egyptian Journal of Petroleum, 26: 405-412.
55. Cao, C.
(1996). On electrochemical techniques for interface inhibitor research. Corrosion Science, 38 (12): 2073-2082.
56. Zachariah,
P. M., Keerthi, R., Cyril, A.,
Bincy, J. and Sam, J. (2020). Corrosion inhibition of mild
steel using poly (2-ethyl -2-oxazoline) in 0.1M HCl solution. Heliyon, 6(11): 1-8.
57.
Poornima, T., Nayak, J. and
Shetty, A. N.
(2012). Effect of diacetyl monoxime thiosemicarbazone on the corrosion of aged 18 Ni 250 grade maraging steel in sulphuric acid solution. Journal of Metallurgy, 2012: 1-13.
58.
Okafor, P. C. and Zheng, Y. (2009). Synergistic inhibition behaviour
of methylbenzyl quaternary imidazoline
derivative and iodide ions on mild steel in H2SO4 solutions. Corrosion
Science, 51: 850-859.
59.
Shukla, S. K. and Quraishi, M.
A. (2010). The effects of pharmaceutically active compound doxycycline on the corrosion of mild steel in hydrochloric acid solution.
Corrosion Science, 52: 314-321.
60. Okafor,
P. C., Ikpi, M. E., Uwah, I. E., Ebenso, E. E., Ekpe, U. J. and Umoren, S. A. (2008). Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corrosion Science, 50 (8): 2310-2317.
61.
Kassim, K.,
Kamal, N. K. M. and Fadzil, A. H. (2016). Synthesis, characterization and
electrochemical studies of 4-methoxybenzoylthiourea derivatives. Malaysian Journal
of Analytical Sciences, 20(6): 1311-1317.
62. Verma,
C., Olasunkanmi, L. O., Obot, I. O., Ebenso, E. E. and Quraishi, M. A. (2016).
5-Arylpyrimido-[4,5-b] quinoline-diones as new and sustainable corrosion
inhibitors for mild steel in 1 M HCl: a combined experimental and theoretical
approach. RSC Advances, 6(19):
15639-15654.
63.
Sourav, K.
S., Alokdut, D., Pritam, G., Dipankar, S. and Priyabrata,
B. (2016). Novel Schiff-base molecules as efficient corrosion inhibitors for mild
steel surface in 1 M HCl medium: experimental and theoretical approach. Physical
Chemistry Chemical Physics, 18(27):
17898-17911.
64.
Fouda, A. S., El‑Desoky, H. S., Abdel‑Galeil, M. A. and
Dina, M. (2021). Niclosamide and dichlorphenamide: new
and effective corrosion inhibitors for carbon steel in 1M HCl
solution. SN Applied Sciences,
3(287): 1-20.
65.
Ghulamullah,
K., Wan, J. B., Salim, N. K., Pervaiz, A., Ladan, M., Ahmed, S.
M., Khan, G. M., Rehman, M. A. and Mohamad Badry, A. B. (2017). Electrochemical
investigation on the corrosion inhibition of mild steel by Quinazoline Schiff
base compounds in hydrochloric acid solution. Journal of Colloid and Interface Science, 502: 134-145.
66.
Sahin, M., Bilgic, S. and Yilmaz, H. (2002). The inhibition effects of
some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Applied Surface Science, 195(1-4): 1-7.
67.
Nazir, U., Akhter, Z., Janjua, N. K., Asghar, M.
A., Kanwal, S., Butt, T. M., Sani, A., Liaqat,
F., Hussain, R. and Shah, F. U. (2020). Biferrocenyl Schiff
bases as efficient corrosion inhibitors for an aluminium alloy in HCl solution:
a combined experimental and theoretical study. RSC Advances, 10: 7585-7599.
68.
Prabakaran, M., Kim, S. H., Hemapriya, V., Gopiraman, M., Kim, I. S.
and Chung, I. M. (2016). Rhus vernicifua as a green corrosion inhibitor for
mild steel in 1 M H2SO4. RSC Advances, 6(62): 57144-57153.
69. Goulart, C. M., Esteves-Souza, A., Martinez-Huitle, C.
A., Rodrigues, C. J. F., Maciel, M. A. M. and Echevarria, A. (2013). Experimental
and theoretical evaluation of semicarbazones and thiosemicarbazones as organic
corrosion inhibitors. Corrosion Science,
67: 281-291.
70. Fathabadi,
H. E., Ghorbani, M. and Ghartavol, H. M. (2021). Corrosion inhibition of mild
steel with tolyltriazole. Materials
Research, 24(4): 1-16.
71.
Ehteshamzadeh, M., Jafari, A. H., Naderi, E. and Hosseini, M. G. (2009). Effect of carbon steel
microstructures and molecular structure of two new Schiff base compounds on
inhibition performance in 1 M HCl solution by EIS. Materials Chemistry and Physics, 113(2-3): 986-993.
72. Govindaraju,
K. M., Gopi, D. and Kavitha, L. (2009). Inhibiting effects of
4-amino-antipyrine based schiff base derivatives on the corrosion of mild steel
in hydrochloric acid. Journal of Applied
Electrochemistry, 39: 2345-2352.
73.
Satapathy, A. K., Gunasekaran, G., Sahoo, S. C., Amit, K. and Rodrigues, P. V. (2009). Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution.
Corrosion Science, 51(12):
2848-2856.
74.
Ebenso, E. E., Arslan, T., Kandemirli,
F., Caner, N. and Love, I. (2010). Quantum chemical studies of some rhodanine
azosulpha drugs as corrosion inhibitors for mild steel in acidic medium. International
Journal of Quantum Chemistry, 110(5):
1003-1018.
75. Solmaz,
R. (2010). Investigation of the inhibition effect of
5-((E)-4-phenylbuta-1,3-dienylideneamino)-1,3,4-thiadiazole-2-thiol Schiff base
on mild steel corrosion in hydrochloric acid. Corrosion Science, 52 (10): 3321-3330.
76.
Masahiko, T., Kazushige, I., Yoichi, W., Motohiro, A. and Motomasa, F.
(2009). Study of polarization curve measurement method for type 304 stainless
steel in BWR high temperature-high purity water. Journal of Nuclear Science and Technology, 46(2): 132-141.
77. Chakravarthy,
M. P., Mohana, K. N. and Pradeep Kumar, C. B. (2014). Behaviour of
nicotinamide derivatives on mild steel in hydrochloric acid solution. International Journal of Industrial
Chemistry, 5 (19): 1-21.
78.
Lorenz, W. J. and Mansfeld, F. (1982). Determination of corrosion
rates by electrochemical DC and AC methods. Corrosion
Science, 21(9-10): 647-672.
79. Youcef,
B., Fatiha, B. and Saida, K. (2021). A new corrosion inhibitor for steel rebar
in concrete: Synthesis, electrochemical and theoretical studies. Journal of Molecular Structure, 1225:
1-17.
80.
Abdelghani, M., Lakhdar, S., Abdelkader,
H., Ilhem, K. and Embarek,
B. (2021). Synthesis, density functional theory study,
molecular dynamics simulation and anti-corrosion performance of two benzidine
Schiff bases. Journal of Molecular
Structure, 1235: 1-15.
81. Merah, S.,
Larabi, L., Abderrahim, O. and Harek, Y. (2017). Study of corrosion inhibition
of C38 steel in 1 M HCl solution by polyethyleneiminemethylene phosphonic acid.
International Journal of Industrial
Chemistry, 8: 263-272.
82. Xifeng, Y., Feng, L. and Weiwei, Z. (2019). 4-(Pyridin-4-yl) thiazol-2-amine as an efficient non-toxic inhibitor
for mild steel in hydrochloric acid solutions. RSC Advances,
9: 10454-10464.
83.
Laabaissi, T., Benhiba, F., Missioui, M., Rouifi, Z., Rbaa, M., Oudda, H., Ramli, Y., Guenbour, A., Warad, I. and Zarrouk, A. (2020). Coupling of chemical, electrochemical and theoretical
approach to study the corrosion inhibition of mild steel by new quinoxaline
compounds in 1 M HCl. Heliyon,
6(5): 1-15.
84.
Quy, H. D., Tran, D. and Nam, P. C. (2021). A Study of
1-benzyl-3-phenyl-2-thiourea as an effective steel corrosion inhibitor in
1.0 M HCl Solution. Journal of
Chemistry, 2021: 1-14.
85.
Parul, D., Quraishi, M. A. and Obot, I. B. (2018). A combined
electrochemical and theoretical study of pyridine-based Schiff bases as novel
corrosion inhibitors for mild steel in hydrochloric acid medium. Journal of Chemical Sciences, 130(8):
1-19.
86. Kumari,
P. P., Shetty, P. and Rao, S. A. (2017). Electrochemical measurements for the
corrosion inhibition of mild steel in 1 M hydrochloric acid by using an
aromatic hydrazide derivative. Arabian
Journal of Chemistry,10(5): 653-663.
87. Maryam,
C., Abdelkarim, C., Hassane, L., Rachid, S., Santosh, L. G., Bhat, K. S.,
Riadh, M., Ismat, H. A., Mohammad, I. K., Hiroki, S. and Ill-Min, C. (2020).
Synthesis and corrosion inhibition evaluation of a new schiff base hydrazone
for mild steel corrosion in HCl medium: electrochemical, DFT, and molecular
dynamics simulations studies. Journal of Adhesion Science and
Technology, 34(12): 1283-1314.
88.
Zhenzhen, Z., Min, S., Yiming, J., Li, L. and Jin, L. (2016). Effect
of tin on the corrosion resistance of 16 Cr ferritic stainless steel in acidic
solution and chloride-containing media. International
Journal of Electrochemical Science, 11: 3963-3975.
89. Zesheng,
C., Zheng, L., Kun-Huan, H., Guo-Cheng, H., Yiju, L., Jiaxing, H. and Xianmei,
W. (2021). Two diamine Schiff base as a corrosion inhibitor for carbon steel in
sulfuric acid solution: Electrochemical assessment and theoretical calculation. International Journal of Electrochemical
Science, 16: 1-21.
90.
Al-Amiery, A. A., Kassim, F. A., Kadhum, A. A. H. and
Mohamad, A. B. (2016). Synthesis and characterization of a novel
eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric
acid. Scientific Reports, 6: 1-13.
91.
Negm, N. A., Kandile, N. G., Badr, E. A. and Mohammed, M. A. (2012). Gravimetric and
electrochemical evaluation of environmentally friendly nonionic corrosion
inhibitors for carbon steel in 1 M HCl. Corrosion Science, 65: 94-103.
92. Narvaez,
L., Cano, E. and Bastidas, D. M. (2005). 3-Hydroxybenzoic acid as AISI 316L
stainless steel corrosion inhibitor in a H2SO4–HF–H2O2
pickling solution. Journal of Applied
Electrochemistry, 35: 499-506.
93.
Ozkır, D., Kayakırılmaz, K., Bayol, E., Gürten, A. A.
and Kandemirli, F. (2012). The inhibition effect of Azure A on mild steel in 1 M HCl. A
complete study: Adsorption, temperature, duration and quantum chemical aspects.
Corrosion Science, 56: 143-152.
94. Agrawal,
R. and Namboodhiri, T. K. G. (1990). The inhibition of sulphuric acid corrosion
of 410 stainless steel by thioureas. Corrosion
Science, 30(1): 37-52.
95. Zhao, T.
P. and Mu, G. N. (1999). The adsorption and corrosion inhibition of anion
surfactants on aluminium surface in hydrochloric acid. Corrosion Science, 41: 1937-1944.
96.
Yaro, A. S., Khadom, A. A. and Ibraheem, H. F. (2011). Peach juice as
an anticorrosion inhibitor of mild steel. Anti-Corrosion
Methods and Materials, 58(3): 116-124.
97.
Hegazy,
M. A., Hasan, A. M., Emara, M. M., Bakr,
M. F. and Youssef, A. H.
(2012). Evaluating four synthesized Schiff
bases as corrosion inhibitors on the carbon steel in 1 M hydrochloric acid. Corrosion Science, 65: 67-76.
98.
Goulart, C. M., Esteves-Souza, A.,
Martinez-Huitle, C. A., Rodrigues, C. J. F., Maciel, M. A. M. and Echevarria,
A. (2013). Experimental
and theoretical evaluation of semicarbazones and thiosemicarbazones as organic
corrosion inhibitors. Corrosion Science,
67 (3): 281-291.
99.
Muthukrishnan, P., Jeyaprabha, B. and Prakash, P. (2017). Adsorption
and corrosion inhibiting behavior of Lannea
coromandelica leaf extract on mild steel corrosion. Arabian Journal of Chemistry, 10: 2343-2354.
100. Adewuyi, A., Gopfert, A. and Wolf, T. (2014).
Succinyl amide gemini surfactant from Adenopus
breviforus seed oil: A potential corrosion inhibitor of mild steel in
acidic medium. Industrial Crops and
Products, 52: 439-449.
101. Ji, G., Shukla, S. K., Dwivedi, P., Sundaram,
S. and Ebenso, E. E. (2012). Green Capsicum
annuum fruit extract for inhibition of mild steel corrosion in hydrochloric
acid solution. International Journal of
Electrochemical Science, 7: 12146-12158.
102. Gopiraman, M., Selvakumaran, N., Kesavan, D.
and Karvembu, R. (2012). Adsorption and corrosion inhibition behaviour of
N-(phenylcarbamothioyl) benzamide on mild steel in acidic medium. Progress in Organic Coatings, 73 (1):
104-111.
103. Ahamad, I., Prasad, R. and Quraishi, M. A.
(2010). Inhibition of mild steel corrosion in acid solution by Pheniramine
drug: experimental and theoretical study. Corrosion
Science, 52 (9): 3033-3041.
104. Fazayel, A. S., Khorasani, M. and Sarabi, A.
A. (2018). The effect of functionalized polycarboxylate structures as corrosion
inhibitors in a simulated concrete pore solution. Applied Surface Science, 441: 895-913.
105. Singh, A. K. and Quraishi, M. A. (2010). Inhibiting
Effects of 5-Substituted Isatin-Based Mannich Bases on the Corrosion of Mild
Steel in Hydrochloric Acid Solution. Journal
of Applied Electrochemistry, 40 (7): 1293-1306.
106. Yurt, A., Bereket, G., Kivrak,
A., Balaban, A. and Erk, B.
(2005). Effect of Schiff bases containing pyridyl group as corrosion inhibitors
for low carbon steel in 0.1 M HCl. Journal
of. Applied Electrochemistry, 35: 1025-1032.
107. Saliyan, V. R. and Adhikari, A. V.
(2008). Quinolin-5-ylmethylene-3-{[8-(trifluoromethyl)quinolin-4-yl]
thio}propanohydrazide as an effective inhibitor of mild steel corrosion in HCl
solution. Corrosion Science, 50 (1): 55-61.
108. Deyab, M.
A. (2015). Egyptian licorice extract as a green corrosion inhibitor for copper
in hydrochloric acid solution. Journal of
Industrial and Engineering Chemistry, 22: 384-389.
109. Abd
El-Lateef, H. M., Abu-Dief, A. M., Abdel-Rahman, L. H., Sanudo, E. C. and Aliaga-Alcalde, N. (2015).
Electrochemical and theoretical quantum approaches on the inhibition of C1018
carbon steel corrosion in acidic medium containing chloride using some newly
synthesized phenolic Schiff bases compounds. Journal of Electroanalytical Chemistry, 743: 120-133.
110. Sigircik, G., Tuken, T. and Erbil, M. (2015).
Inhibition effectiveness of aminobenzonitrile compounds on steel surface. Applied Surface Science, 324: 232-239.
111. Kowsari, E., Payami, M., Amini, R.,
Ramezanzadeh, B. and Javanbakht, M. (2014). Task-specific ionic liquid as a new
green inhibitor of mild steel corrosion. Applied
Surface Science, 289: 478-486.
112. Zhang, H. H., Qin, C. K., Chen, Y. and Zhang,
Z. (2019) Inhibition behaviour of mild steel by three new benzaldehyde
thiosemicarbazone derivatives in 0.5 M H2SO4:
Experimental and computational study. Royal
Society Open Science, 6(8): 1-19.
⇚
⤊ ⇛
