Malaysian
Journal of Analytical Sciences Vol 26 No 3
(2022): 492 - 506
SYNTHESIS OF GREEN-RENEWABLE BIOLUBRICANT BASE
STOCK FROM MALAYSIAN PALM OIL
(Sintesis
Stok Asas Biopelincir Hijau-Diperbaharui daripada Minyak Sawit Malaysia)
Nurazira Mohd Nor1* and Jumat Salimon2
1MaterOleo
Research Group,
Faculty
of Applied Sciences,
Universiti
Teknologi MARA, Cawangan Negeri Sembilan, Kampus Kuala Pilah, 72000 Kuala
Pilah, Negeri Sembilan, Malaysia
2Department
of Chemical Sciences, Faculty of Science and Technology,
Universiti
Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
*Corresponding author: nurazira@uitm.edu.my
Received: 20 August 2021; Accepted: 3 February 2022; Published: 27 June 2022
Abstract
Palm oil has become one of the potential
renewable resources in biolubricant application. However, the direct
application of palm oil as a biolubricant is restricted due to its low
oxidative stability and poor low temperature properties. These drawbacks can be
overcome by molecule structural redesign through chemical modification process.
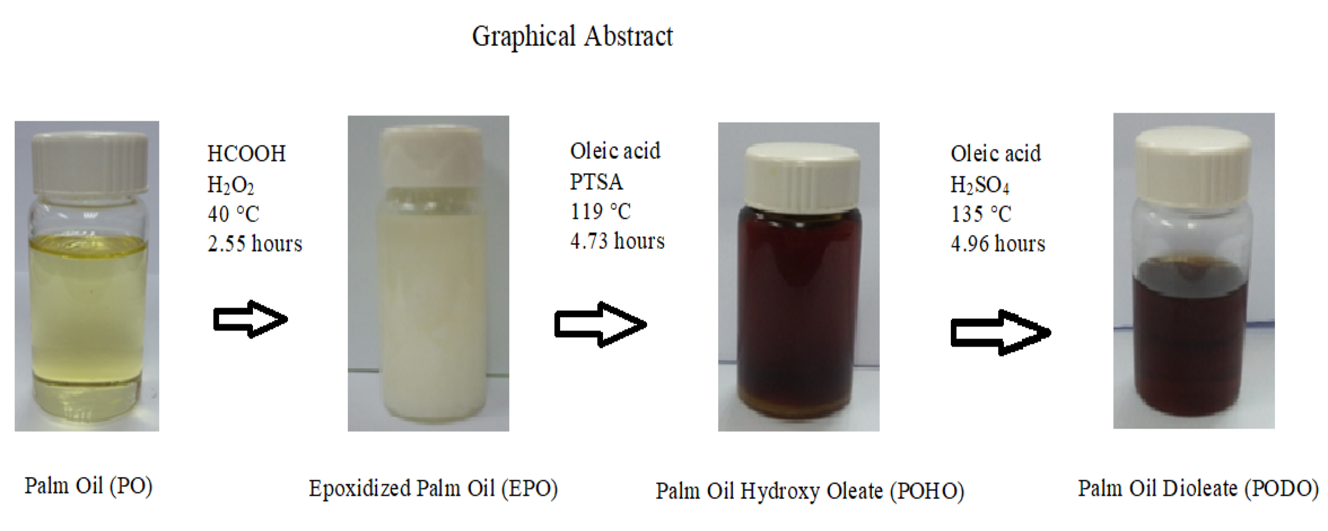
Palm oil (PO) was modified via epoxidation, ring opening and esterification
process. The epoxidized palm oil (EPO) was prepared by using an in-situ
performic acid catalyst. Then, EPO was ring-opened using oleic acid in the
presence of p-toluenesulfonic acid (PTSA) as a catalyst and further
esterification with oleic acid using sulfuric acid as catalyst. The molecular structure confirmation of
modified palm oils was proven through the oxirane oxygen content (OOC) value,
iodin value, hydroxyl value, Fourier transformation infra-red (FTIR), proton
and carbon nuclear magnetic resonance (1H-NMR and 13C-NMR)
spectroscopy analysis. Results showed that the conversion of PO into EPO
has improved its oxidative stability (190 °C).
While, the esterification process has resulted in branching and bending in the
molecule structure of the final product (palm oil dioleate, PODO), which
improved its pour point (-10 °C), flash point (315 °C) and viscosity index
(146). These make PODO suitable to be used in biolubricant application.
Keywords: biolubricant,
epoxidation, esterification, ring opening, palm oil
Abstrak
Minyak
sawit merupakan salah satu sumber yang boleh diperbaharui yang berpotensi dalam
penghasilan biopelincir. Walaubagaimanapun, penggunaan secara terus minyak
sawit sebagai biopelincir adalah terhad disebabkan oleh kestabilan oksidatif
yang rendah dan sifat suhu rendah yang lemah. Kelemahan ini boleh diatasi
dengan ubahsuai struktur molekul melalui proses pengubahsuaian kimia. Minyak
sawit (PO) diubahsuai melalui proses pengepoksidaan, pembukaan gelang dan
pengesteran. Minyak sawit terepoksida (EPO) dihasilkan menggunakan mangkin asid
performik yang dijana secara in-situ. Seterusnya, EPO ditindakbalaskan melalui
pembukaan gelang menggunakan asid oleik dengan kehadiran p-toluena asid
sulfonik (PTSA) sebagai mangkin dan diikuti dengan pengesteran dengan asid
oleik dengan menggunakan mangkin asid sulfurik. Pengecaman struktur molekul
minyak sawit terubahsuai dibuktikan melalui nilai kandungan oksigen oksiran,
nilai iodin, nilai hidroksil, spektroskopi infra-merah transformasi Fourier
(FTIR), proton dan karbon nuklear magnetik resonans (1H-NMR dan 13C-NMR).
Keputusan menunjukkan penukaran PO kepada EPO telah memperbaiki nilai
kestabilan oksidatifnya (190 °C). Manakala proses pengesteran telah
menghasilkan struktur molekul yang bercabang dan bengkok bagi hasil akhir
(dioleate minyak sawit, PODO) dan telah memperbaiki nilai takat tuang (-10 °C),
takat kilat (315 °C) dan indeks kelikatan (146). Ini menjadikan PODO sesuai
untuk digunakan dalam aplikasi biopelincir.
Kata kunci: biopelincir,
pengepoksidaan, pengesteran, pembukaan gelang, minyak sawit
Graphical Abstract
References
1.
Salimon, J. and Salih, N.
(2009). Oleic acid diesters: Synthesis,
characterization and low temperature properties. European Journal of Scientific Research, 32(2): 216-222.
2.
Farhoosh, R., Einafshar,
S. and Sharayei, P. (2009). The effect of commercial refining steps on the
rancidity measures of soybean and canola oils. Food Chemistry, 115:
933-938.
3.
Erhan, S. Z. and
Asadauskas, S. (2000). Lubricant basestocks from vegetable oils. Industrial Crops and Products, 11:
277-282.
4.
Nirmal, V. P. and
Dineshbabu, D. (2015). Performance and emission of Pongamia pinnata oil as a lubricant in diesel engine. International Journal of Innovative Research
in Science, Engineering and Technology, 4(2): 75.
5.
Salih, N. and Salimon, J.
(2021). A review on eco-friendly green biolubricants from renewable and
sustainable plant oil sources. Biointerface Research in Applied Chemistry,
11(5): 13303-13327.
6.
Salimon, J., Salih, N.
and Yousif, E. (2011). Chemically modified biolubricant basestocks from
epoxidized oleic acid: Improved low temperature properties and oxidative
stability. Journal of Saudi Chemical
Society, 15: 195-201.
7.
Wu, X., Zhang, X., Yang,
S., Chen, H. and Wang, D. (2000). The study of epoxidized rapeseed oil used as
a potential biodegradable lubricant. Journal of the American Oil Chemist
Society, 77: 561-563.
8.
Salimon, J. and Salih, N.
(2010). Modification of epoxidized ricinoleic acid for biolubricant base oil
with improved flash and pour points. Asian
Journal of Chemistry, 22(7): 5468-5476.
9.
Adhvaryu,
A., Liu, Z. and Erhan, S. Z. (2005). Synthesis of novel alkoxylated
triacylglycerols and their lubricant base oil properties. Industrial Crops
and Products, 21:113-119.
10.
Borugadda, V. B. and
Goud, V. V. (2015). Response surface methodology for optimization of
biolubricant basestock synthesis from high free fatty acids castor oil. Energy
Science & Engineering, 3(4): 371-383.
11.
Erhan, S. Z., Sharma,
B.K. and Perez, J. M. (2006). Oxidation and low temperature stability of
vegetable oil-based lubricants. Industrial Crops and Products, 24:
292-299.
12.
Sharma, B. K., Perez, J.
M. and Erhan, S. Z. (2007). Soybean oil-based lubricants: A search for
synergistic antioxidants. Energy Fuels, 21: 2408-2414.
13.
Singh, C. P. and
Chhibber, V. K. (2013). Chemical modification in karanja oil for biolubricant
industrial applications. Journal Drug Delivery Therapeutics, 3:117-122.
14.
Borugadda, V. B. and
Goud, V. V. (2013). Comparative studies of thermal, oxidative and low
temperature properties of waste cooking oil and castor oil. Journal
Renewable Sustainable Energy, 5: 063104.
15.
Salimon, J. and Salih, N.
(2009). Substituted esters of octadecanoic acid as potential biolubricants. European
Journal of Scientific Research, 31(2): 273 – 227.
16.
Moser, B. R. and Erhan,
S. Z. (2007). Preparation and evaluation of a series of α-hydroxy ethers
from 9, 10-epoxystreates. European Journal Lipid Science Technology, 109:
206-213.