Malaysian
Journal of Analytical Sciences Vol 26 No 3
(2022): 613 - 621
PtRu SUPPORTED ON POROUS 3D TITANIUM DIOXIDE-GRAPHENE AEROGEL
AS A POTENTIAL ELECTROCATALYST FOR DIRECT METHANOL FUEL CELLS
(PtRu disokong pada 3D Titanium Dioksida-Grafin Aerogel
Berliang Sebagai Potensi Elektromangkin untuk Sel Bahan Bakar Metanol Langsung)
Siti Hasanah Osman1*, Siti
Kartom Kamarudin1,2, Sahriah Basri1, Nabila A.Karim1
1Fuel Cell Institute
2Department of Chemical and Process
Engineering, Faculty of Engineering and Built Environment
Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor,
Malaysia
*Corresponding author: ctie@ukm.edu.my
Received: 9 November 2021; Accepted: 27 February 2022; Published: 27 June 2022
Abstract
The catalyst support is typically implemented to
improve the catalytic activity in direct methanol fuel cells (DMFCs). Thus,
this study focused on the novel support of 3D hierarchical porous TiO2-graphene
aerogel which was established via a combination of hydrothermal method and
freezing drying method. XRD, Raman spectra, and FESEM were used to study the
PtRu/TiO2-GA. The estimated particle size of PtRu/TiO2-GA
determined from the XRD analysis was less than composite TiO2-GA. The existence of the
carbon support material was confirmed by the Raman spectra in all generated
samples. Within the electrocatalyst and TiO2-GA, the ratio value of
the D band to the G band (ID/IG) was not significantly
different. The computed ID/IG values for TiO2-GA
and PtRu/TiO2-GA electrocatalysts were 0.99 and 1.02, respectively. The best TiO2-GA was doped with PtRu
catalyst for the electrochemical test and DMFC performance based on FESEM
characterization. PtRu/ TiO2-GA exhibited better electrocatalytic
activity, as well as improved PtRu usage efficiency stability and methanol
oxidation reaction. Notably, the ECSA value was around 76.01 m2g-1,
and the mass activity (957.15 mAmg-1) was higher than commercial
with the same loading (20%) PtRu/C (110.79 mAmg-1). Interestingly after the 2000s, the
current density of PtRu/TiO2-GA was consistently higher than that of PtRu/C. The
superior electrocatalytic performance of PtRu/TiO2-GA
towards methanol oxidation
demonstrates its use in practical application as a promising anode material for
DMFCs
Keywords: 3D titanium dioxide-graphene aerogel,
platinum-ruthenium nanoparticles, electrocatalysis, methanol electro-oxidation
Abstrak
Sokongan mangkin biasanya
dilaksanakan untuk meningkatkan aktiviti pemangkin dalam sel bahan api metanol
langsung (SFML). Oleh itu, kajian ini memberi tumpuan kepada novel sokongan
pada TiO2-grafin aerogel berliang hierarki 3D telah ditubuhkan
dengan gabungan kaedah hidroterma dan kaedah pengeringan beku. XRD, spektrum
Raman dan FESEM digunakan untuk mengkaji PtRu/TiO2-GA. Anggaran saiz
zarah PtRu/TiO2-GA yang ditentukan daripada analisis XRD adalah
kurang daripada komposit TiO2-GA. Kewujudan bahan sokongan karbon
telah disahkan oleh spektrum Raman dalam semua sampel yang dihasilkan. Dalam elektromangkin
dan TiO2-GA, nilai nisbah jalur D kepada jalur G (ID/IG)
tidak berbeza dengan ketara. Nila ID/IG yang dikira untuk
elektromangkin TiO2-GA dan PtRu/TiO2-GA ialah 0.99 dan
1.02, masing-masing. TiO2-GA terbaik akan didop dengan mangkin PtRu
untuk ujian elektrokimia dan prestasi SFML berdasarkan pencirian FESEM. PtRu/
TiO2-GA mempamerkan aktiviti elektrokatalitik yang lebih baik, serta
peningkatan kestabilan kecekapan penggunaan PtRu dan tindak balas pengoksidaan
metanol. Terutama, nilai ECSA adalah sekitar 76.01 m2g-1,
aktiviti jisim (957.15 mAmg-1) adalah lebih tinggi daripada
komersial dengan pemuatan yang sama (20%) PtRu/C (110.79 mAmg-1).
Menariknya selepas minit ke-2000 saat, ketumpatan semasa PtRu/TiO2-GA
sentiasa lebih tinggi daripada PtRu/C. Prestasi elektrokatalitik unggul
PtRu/TiO2-GA terhadap pengoksidaan metanol boleh digunakan dalam
aplikasi praktikal sebagai bahan anod yang menjanjikan untuk DMFC.
Kata kunci: 3D titanium dioksida-grafin aerogel, nanopartikel
platinum-ruthenium, elektromangkin, elektro-pengoksidaan metanol
Graphical Abstract
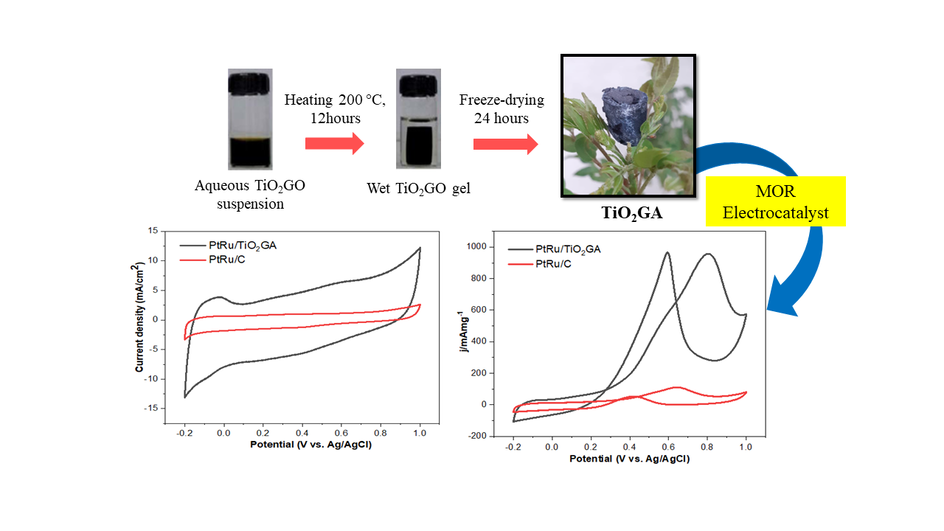
References
1.
Rashidi, R., Dincer, I., Naterer, G. F. and Berg, P.
(2009). Performance evaluation of direct methanol fuel cells for portable
applications. Journal of Power Sources, 187(2): 509-516.
2.
Achmad,
F., Kamarudin, S. K., Daud, W. R. W. and Majlan, E. H. (2011). Passive direct
methanol fuel cells for portable electronic devices. Applied Energy, 88(5):
1681-1689.
3.
Zhao,
L., Wang, Z. B., Li, J. L., Zhang, J. J., Sui, X. L. and Zhang, L. M. (2016).
Hybrid of carbon-supported Pt nanoparticles and three dimensional graphene
aerogel as high stable electrocatalyst for methanol electrooxidation. Electrochimica
Acta, 189: 175-183.
4.
Bock,
C., Paquet, C., Couillard, M., Botton, G. A. and MacDougall, B. R. (2004).
Size-selected synthesis of PtRu nano-catalysts: reaction and size control
mechanism. Journal of the American Chemical Society, 126(25): 8028-8037.
5.
Zhang,
X., Ma, J., Yan, R., Cheng, W., Zheng, J. and Jin, B. (2021).
Pt-Ru/polyaniline/carbon nanotube composites with three-layer tubular structure
for efficient methanol oxidation. Journal of Alloys and Compounds, 867:
159017.
6.
Li, W.,
Wang, Q., Wang, L., Fu, X. Z. and Luo, J. L. (2021). Mesoporous CeO2–C
hybrid spheres as efficient support for platinum nanoparticles towards methanol
electrocatalytic oxidation. Journal of Rare Earths, 39(6): 674-681.
7.
Yang,
Y., Guo, Y. F., Fu, C., Zhang, R. H., Zhan, W., Wang, P., ... & Zhou, X. W.
(2021). In-situ loading synthesis of graphene supported PtCu nanocube and its
high activity and stability for methanol oxidation reaction. Journal of
Colloid and Interface Science, 595: 107-117.
8.
Ren,
X., Lv, Q., Liu, L., Liu, B., Wang, Y., Liu, A. and Wu, G. (2020). Current
progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustainable
Energy & Fuels, 4(1): 15-30.
9.
Karuppanan,
K. K., Raghu, A. V., Panthalingal, M. K., Thiruvenkatam, V., Karthikeyan, P.
and Pullithadathil, B. (2019). 3D-porous electrocatalytic foam based on Pt@
N-doped graphene for high performance and durable polymer electrolyte membrane
fuel cells. Sustainable Energy & Fuels, 3(4): 996-1011.
10.
Gorgolis,
G. and Galiotis, C. (2017). Graphene aerogels: a review. 2D Materials,
4(3): 032001.
11.
Mao,
J., Iocozzia, J., Huang, J., Meng, K., Lai, Y. and Lin, Z. (2018). Graphene
aerogels for efficient energy storage and conversion. Energy &
Environmental Science, 11(4): 772-799.
12.
Siwińska-Stefańska,
K. and Jesionowski, T. (2017). Advanced hybrid materials based on titanium
dioxide for environmental and electrochemical applications. In Titanium
Dioxide. IntechOpen.
13.
Odling,
G. and Robertson, N. (2015). Why is anatase a better photocatalyst than rutile?
The importance of free hydroxyl radicals. ChemSusChem, 8(11): 1838-1840.
14.
Dey, S.
and Mehta, N. S. (2020). Synthesis and applications of titanium oxide catalysts
for lower temperature CO oxidation. Current Research in Green and
Sustainable Chemistry, 3: 10002.
15.
Bagheri,
S., Muhd Julkapli, N. and Bee Abd Hamid, S. (2014). Titanium dioxide as a
catalyst support in heterogeneous catalysis. The Scientific World Journal, 2014:
727496.
16.
Abdullah,
N., Kamarudin, S. K. and Shyuan, L. K. (2018). Novel anodic catalyst support
for direct methanol fuel cell: characterizations and single-cell performances. Nanoscale
Research Letters, 13(1): 1-13.
17.
Ito,
Y., Takeuchi, T., Tsujiguchi, T., Abdelkareem, M. A. and Nakagawa, N. (2013).
Ultrahigh methanol electro-oxidation activity of PtRu nanoparticles prepared on
TiO2-embedded carbon nanofiber support. Journal of Power Sources,
242: 280-288.
18.
Ercelik,
M., Ozden, A., Seker, E. and Colpan, C. O. (2017). Characterization and
performance evaluation of PtRu/CTiO2 anode electrocatalyst for DMFC
applications. International Journal of Hydrogen Energy, 42(33):
21518-21529.
19.
Liu,
R., Guo, W., Sun, B., Pang, J., Pei, M. and Zhou, G. (2015). Composites of
rutile TiO2 nanorods loaded on graphene oxide nanosheet with enhanced
electrochemical performance. Electrochimica Acta, 156: 274-282.
20.
Xiang,
C., Guo, R., Lan, J., Jiang, S., Wang, C., Du, Z. and Cheng, C. (2018).
Self-assembling porous 3D titanium dioxide-reduced graphene oxide aerogel for
the tunable absorption of oleic acid and RhodamineB dye. Journal of Alloys
and Compounds, 735: 246-252.
21.
Cordero-Borboa,
A. E., Sterling-Black, E., Gómez-Cortés, A. and Vázquez-Zavala, A. (2003).
X-ray diffraction evidence of the single solid solution character of
bi-metallic Pt-Pd catalyst particles on an amorphous SiO2 substrate.
Applied Surface Science, 220(1-4): 169-174.
22.
Vorokh,
A. S. (2018). Scherrer formula: estimation of error in determining small
nanoparticle size. Nanosystem Physics Chemistry Mathematics, pp.
364-369.
23.
Stankovich,
S., Dikin, D. A., Piner, R. D., Kohlhaas, K. A., Kleinhammes, A., Jia, Yue, W.,
SonBinh, T. N., and Ruoff, R. S. (2007). Synthesis of graphene-based nanosheets
via chemical reduction of exfoliated graphite oxide. carbon, 45(7): 1558-1565.
24.
Zhu,
Y., Murali, S., Cai, W., Li, X., Suk, J. W., Potts, J. R. and Ruoff, R. S.
(2010). Graphene and graphene oxide: synthesis, properties, and applications. Advanced
Materials, 22(35): 3906-3924.
25.
Li, Y.,
Cai, Q., Wang, L., Li, Q., Peng, X., Gao, B., … and Chu, P. K. (2016).
Mesoporous TiO2 nanocrystals/graphene as an efficient sulfur host
material for high-performance lithium–sulfur batteries. ACS Applied
Materials & Interfaces, 8(36): 23784-23792.
26.
Norilhamiah,
Y., Kamaruddin, S. K., Karim, N. A.,
Masdar, M. S. and Loh, K. S. (2017). Gliserol, P. E. (2017). Preliminary
study on pd-based binary catalysts supported with carbon nanofiber for the
electrooxidation of glycerol. Malaysian Journal of Analytical Sciences, 21(3):
700-708.
27.
Ibrahim,
S. A., Anwar, M. K., Ainuddin, A. R., Hariri, A., Rus, A. Z. M., Kamdi, Z.,
Yunos, M. Z., and Harun, Z. (2019). Synthesis and characterization of visible
light active Fe-TiO2 using hydrothermal method. International
Journal of Integrated Engineering, 11(5): 80-85.
28.
Shaari,
N. and Kamarudin, S. K. (2019). Current status, opportunities, and challenges
in fuel cell catalytic application of aerogels. International Journal of
Energy Research, 43(7): 2447-2467.
29.
Basri,
S., Kamarudin, S. K., Daud, W. R. W., Yaakob, Z. and Kadhum, A. A. H. (2014).
Novel anode catalyst for direct methanol fuel cells. The Scientific World
Journal, 2014: 547604.
30.
Ramli,
Z. A., Kamarudin, S. K., Basri, S. and Zainoodin, A. M. (2020). The potential
of novel carbon nanocages as a carbon support for an enhanced methanol electro - oxidation
reaction in a direct methanol fuel cell. International Journal of Energy
Research, 44(13): 10071-10086.