Malaysian
Journal of Analytical Sciences Vol 26 No 3
(2022): 589 - 599
THE THERMAL
STABILITY AND PRELIMINARY PERFORMANCE OF SODIUM ALGINATE AND POLYVINYL
ALCOHOL-BASED MEMBRANE IN DMFC: MONTMORILLONITE AS A FILLER
(Kestabilan Haba dan Prestasi Awal Membran
Berasaskan Natrium Alginat dan Polivinil Alkohol untuk DMFC: Montmorillonit
sebagai Pengisi)
Maryam Taufiq Musa and Norazuwana Shaari*
Fuel Cell Institute,
Universiti Kebangsaan Malaysia, Bangi,43600,
Selangor, Malaysia
*Corresponding
author: norazuwanashaari@ukm.edu.my
Received: 15 December 2021; Accepted: 24 March 2022;
Published: 27 June 2022
Abstract
Biopolymer-based membranes have emerged vastly
in recent years for fuel cell applications. Polymer electrolyte membranes (PEM)
have become a major component in DMFC stacks that have gone through many
studies beforehand. An improvement has been made to PEM, especially using
biopolymers like alginate based. Sodium alginate (SA) has significant
characteristics such as being too hydrophilic, which is its biggest weakness as
a PEM. Blending it with another polymer of polyvinyl alcohol (PVA) and adding a
clay filler of montmorillonite (MMT) would be a great solution, in this work.
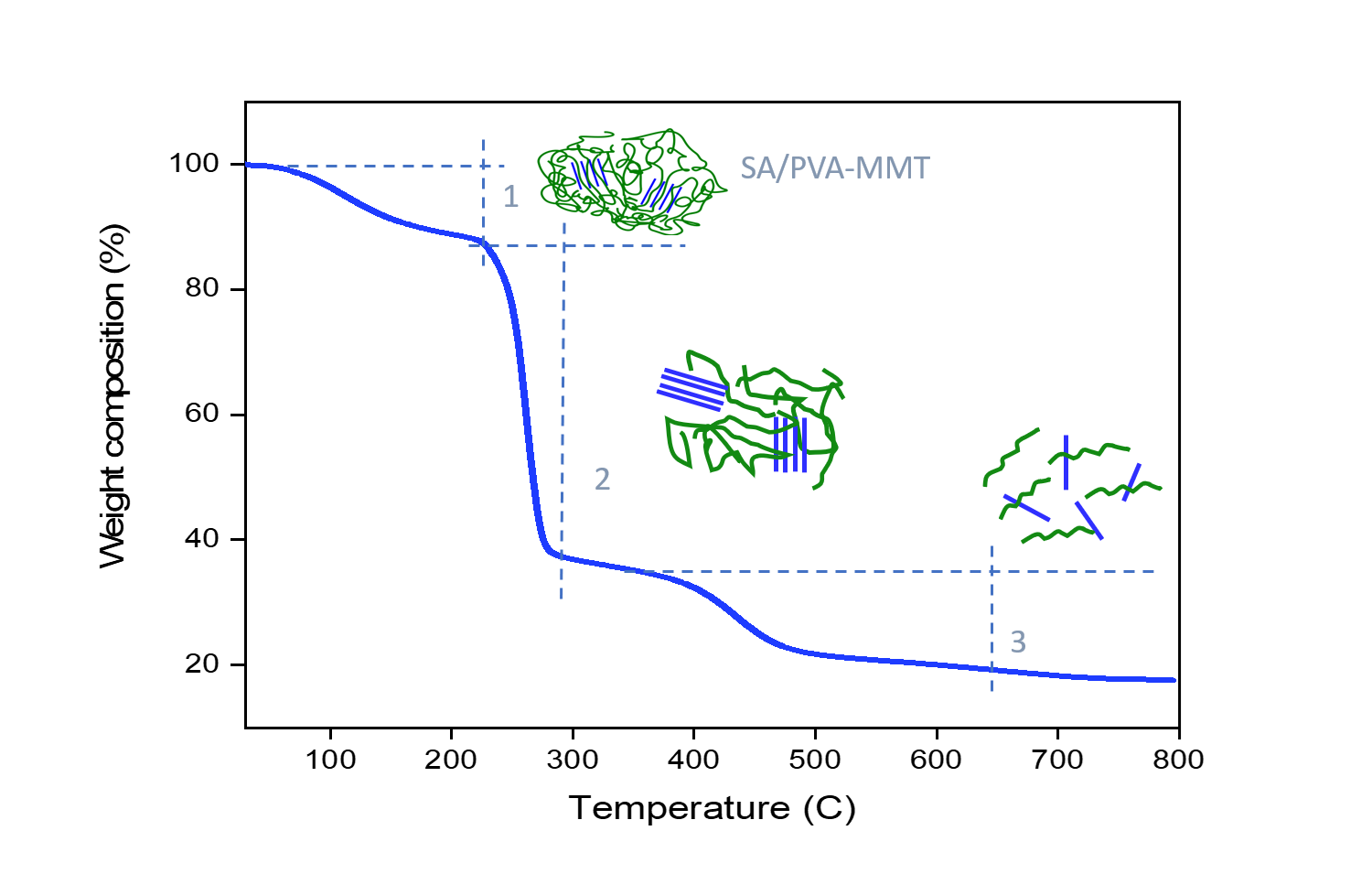
From TGA-DSC analysis, SA/PVA-MMT achieved different thermal stability as the
filler content differed. The glass transition temperature of the membrane had
increased as the MMT content increased, at a maximum range of 240-260 °C at 15
wt.% of MMT content. The membrane’s thermal stability ranking was then followed
by 10 wt.% (250 °C) and 2 wt.% (240 °C) of filler content. The higher the glass
transition temperature, the greater the thermal stability due to the greater
mass loss after being exposed to a later heating. Meanwhile, for the proton
conductivity test, a hybrid membrane of SA/PVA-MMT with SVM 20 (20 wt.% MMT) obtained
the highest value of 8.0510 mS/cm, followed by SVM 10, SVM 2, SVM 15 and SVM 5 with
values of 6.5025, 2.6429, 2.0332 and 1.6083 mS/cm respectively. Higher proton
conductivity enables the potential of the hybrid to conduct electricity in
DMFC. The lowest methanol uptake was shown by the membrane with 20 wt.% MMT
content, with a value of 53.00%, followed by 15 wt.% (97.46%), 10 wt.%
(121.59%), 5 wt.% (132.23%) and 2 wt.% (203.30%), respectively. Low methanol
uptake of the membrane indicated that the DMFC stack could operate with high
efficiency. This study showed that SA/PVA-MMT could be a promising choice of
PEM with an optimum MMT content of 10 wt.% for a better performance produced by
a cheaper hybridized membrane.
Keywords: alginate, polyvinyl alcohol, montmorillonite, copolymer membrane, thermal
stability
Abstrak
Membran berasaskan biopolimer
telah muncul secara meluas sejak kebelakangan tahun ini, dalam aplikasi sel
fuel. Membran polimer elektrolit (PEM) telah menjadi komponen utama dalam tindanan
DMFC, yang mana telah menjadi objek kajian berkali ganda sebelumnya. Satu
penambahbaikan PEM telah dibuat khususnya menggunakan biopolimer alginat. Natrium
Alginat (SA) mempunyai sifat signifikan iaitu terlalu hidrofili, yang mana
ianya merupakan kelemahan terbesar sebagai PEM. Di dalam kajian ini, Natrium
alginat (SA) akan dicampur bersama polivinil alkohol (PVA) dan montmorillonit
(MMT), sejenis pengisi berasaskan tanah liat. Campuran ini telah menghasilkan
prestasi yang amat baik. Daripada analisis TGA-DSC, SA/PVA-MMT telah mencapai
kestabilan haba yang berbeza apabila kandungan pengisi turut berbeza. Suhu peralihan
kaca membran tersebut bertambah apabila kandungan MMT dalam sampel juga
bertambah, pada 15 wt.% MMT, maksima julat suhu dicapai ialah 240-260 ℃.
Kestabilan haba membran ini kemudiannya diikuti dengan 10 wt.% (250 ℃)
dan 2 wt.% (240 ℃) konten pengisi. Semakin tinggi suhu peralihan kaca,
semakin tinggi kestabilan haba membran disebabkan jisim mula berkurang banyak
pada suhu pemanasan yang tinggi (lebih lewat). Manakala untuk kekonduksian
proton, SVM 20 (20 wt.% MMT) mendapat nilai tertinggi iaitu, 8.0510 mS/cm,
diikuti dengan SVM 10, SVM 2, SVM 15 dan SVM 5 dengan nilaian 6.5025, 2.6429, 2.0332
dan 1.6083 mS/cm masing-masing. Tingginya nilai kekonduksian proton, semakin
besarlah potensi membran hibrid tersebut untuk mengalirkan elektrik dalam DMFC.
Ambilan metanol terendah dibuktikan oleh SVM 20 (20 wt.% MMT) dengan nilaian 53.00%,
diikuti dengan 15 wt.% (97.46%), 10 wt.% (121.59%), 5 wt.% (132.23%) dan 2 wt%
(203.30%) masing-masing. Hasil ambilan
metanol yang rendah menunjukkan tindanan DMFC boleh beroperasi pada tahap
keberkesanan yang tinggi. Kajian ini menyimpulkan bahawa SA/PVA-MMT boleh
menjadi pilihan PEM yang terbaik, dengan kandungan MMT optimum pada 10 wt%
beserta kos yang rendah untuk prestasi yang lebih baik.
Kata kunci: alginat, polivinil alkohol, montmorillonit, membran
dwipolimer, kestabilan haba
Graphical Abstract
References
1.
R., Chirachanchai,
S., Shishatskiy, S. and Nunes, S. P. (2008). Sulfonated
montmorillonite/sulfonated poly(ether ether ketone) (SMMT/SPEEK) nanocomposite
membrane for direct methanol fuel cells (DMFCs). Journal of Membrane Science,
323(2), 337-346.
2.
Singha,
S., Koyilapu, R., Dana, K. and Jana, T. (2019). Polybenzimidazole-clay
nanocomposite membrane for PEM fuel cell: Effect of organomodifier structure. Polymer,
167: 13-20.
3.
Yang, C.
C. (2011). Fabrication and characterization of poly(vinyl alcohol)/montmorillonite/
poly(styrene sulfonic acid) proton-conducting composite membranes for direct
methanol fuel cells. International Journal of Hydrogen Energy, 36(7): 4419–4431.
4.
You, P.
Y., Kamarudin, S. K. and Masdar, M. S. (2019). Improved performance of
sulfonated polyimide composite membranes with rice husk ash as a bio-filler for
application in direct methanol fuel cells. International Journal of Hydrogen
Energy, 44(3): 1857-1866.
5.
Xing,
D., He, G., Hou, Z., Ming, P. and Song, S. (2011). Preparation and
characterization of a modified montmorillonite/sulfonated polyphenylether
sulfone/PTFE composite membrane. International Journal of Hydrogen Energy,
36(3): 2177-2183.
6.
Rosli,
N. A. H., Loh, K. S., Wong, W. Y., Mohamad Yunus, R., Lee, T. K., Ahmad, A. and
Chong, S. T. (2020). Review of chitosan-based polymers as proton exchange
membranes and roles of chitosan-supported ionic liquids. International
Journal of Molecular Sciences, 21(632):
1-52.
7.
Mohy
Eldin, M. S., Farag, H. A., Tamer, T. M., Konsowa, A. H. and Gouda, M. H.
(2020). Development of novel iota carrageenan-g-polyvinyl alcohol
polyelectrolyte membranes for direct methanol fuel cell application. Polymer
Bulletin, 77(9), 4895-4916.
8.
Shaari,
N., Kamarudin, S. K. and Zakaria, Z. (2019). Potential of sodium
alginate/titanium oxide biomembrane nanocomposite in DMFC application. International
Journal of Energy Research, 43(14):
8057-8069.
9.
Yang, C.
C. and Lin, S. J. (2002). Preparation of composite alkaline polymer
electrolyte. Materials Letters, 57(4): 873-881.
10.
Charradi,
K., Ahmed, Z., Aranda, P. and Chtourou, R. (2019). Silica / montmorillonite
nanoarchitectures and layered double hydroxide- SPEEK based composite membranes
for fuel cells applications. Applied Clay Science, 174: 77-85.
11.
Sainul
Abidin, K., Kannan, R., Bahavan Palani, P. and Rajashabala, S. (2017). Role of
structural modifications of montmorillonite , electrical properties effect ,
physical behavior of nanocomposite proton conducting membranes for direct
methanol fuel cell applications. Materials Science-Poland, 35(4): 707-716.
12.
Wang,
J., Gong, C., Wen, S., Liu, H., Qin, C., Xiong, C. and Dong, L. (2018). Proton
exchange membrane based on chitosan and solvent-free carbon nanotube fluids for
fuel cells applications. Carbohydrate Polymers, 186: 200-207.
13.
Zakaria,
Z., Kamarudin, S. K., Timmiati, S. N. and Masdar, M. S. (2019). New composite
membrane poly(vinyl alcohol)/graphene oxide for direct ethanol–proton exchange
membrane fuel cell. Journal of Applied Polymer Science, 136(2): 1-13.
14.
Yang, C.
C. and Lee, Y. J. (2009). Preparation of the acidic PVA/MMT nanocomposite
polymer membrane for the direct methanol fuel cell (DMFC). Thin Solid Films,
517(17): 4735-4740.
15.
Wu,
X.-W., Wu, N., Shi, C.-Q., Zheng, Z.-Y., Qi, H.-B. and Wang, Y.-F. (2016).
Proton conductive montmorillonite-Nafion composite membranes for direct ethanol
fuel cells. Journal of Applied Surface Science, 2016: 1-6.
16.
Wang, F.,
Wang, D. and Zhu, H. (2018). Montmorillonite-polybenzimidazole
inorganic-organic composite membrane with electric field-aligned proton
transport channel for high temperature proton exchange membranes. Polymer-Plastics
Technology & Engineering, 2018:
1-8.
17.
Ata, K.
C., Kadioglu, T., Turkmen, A. C., Celik, C. and Akay, R. G. (2020).
Investigation of the effects of SPEEK and its clay composite membranes on the
performance of direct borohydride fuel cell. International Journal of
Hydrogen Energy, 45(8): 5430-5437.
18.
Shaari,
N., Kamarudin, S. K., Basri, S., Shyuan, L. K., Masdar, M. S. and Nordin, D.
(2018). Enhanced mechanical flexibility and performance of sodium alginate
polymer electrolyte bio-membrane for application in direct methanol fuel cell. Journal
of Applied Polymer Science, 135(37):
46666.
19.
Yang, J.
M., Wang, N. C. and Chiu, H. C. (2014). Preparation and characterization of
poly(vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer
electrolytes membrane. Journal of Membrane Science, 457: 139-148.
20.
Shaari,
N. and Kamarudin, S. K. (2020). Sodium alginate/alumina composite biomembrane
preparation and performance in DMFC application. Polymer Testing, 81: 106183.
21.
Yang, C.
C., Lee, Y. J. and Yang, J. M. (2009). Direct methanol fuel cell (DMFC) based
on PVA/MMT composite polymer membranes. Journal of Power Sources, 188(1): 30-37.
22.
Shaari,
N., Kamarudin, S. K., Basri, S., Shyuan, L. K., Masdar, M. S., & Nordin, D.
(2018). Enhanced Proton Conductivity and Methanol Permeability Reduction via
Sodium Alginate Electrolyte-Sulfonated Graphene Oxide Bio-membrane. Nanoscale
Research Letters, 13(1): 1-16.
23.
Hemalatha,
R., Alagar, M., Selvasekarapandian, S., Sundaresan, B. and Moniha, V. (2019).
Studies of proton conducting polymer electrolyte based on PVA, amino acid
proline and NH4SCN. Journal of Science: Advanced Materials and
Devices, 4(1): 101-110.
24.
Kreuer,
K. D. (2001). On the development of proton conducting polymer membranes for
hydrogen and methanol fuel cells. Journal of Membrane Science, 185: 29-39.
25.
Radmanesh,
F., Rijnaarts, T., Moheb, A., Sadeghi, M. and de Vos, W. M. (2019). Enhanced
selectivity and performance of heterogeneous cation exchange membranes through
addition of sulfonated and protonated Montmorillonite. Journal of Colloid and
Interface Science, 533: 658-670.
26.
Uddin,
F. (2018). Montmorillonite: An introduction to properties and utilization. IntechOpen
Web of Science, Pakistan: pp. 1-23.
27.
Kakati,
N., Maiti, J., Das, G., Lee, S. H. and Yoon, Y. S. (2015). An approach of
balancing the ionic conductivity and mechanical properties of PVA based
nanocomposite membrane for DMFC by various crosslinking agents with ionic
liquid. International Journal of Hydrogen Energy, 40(22): 7114-7123.
28.
Yang,
C.-C., Chiu, S. J., Lee, K.-T., Chien, W.-C., Lin, C.-T. and Huang, C.-A.
(2008). Study of poly(vinyl alcohol)/titanium oxide composite polymer membranes
and their application on alkaline direct alcohol fuel cell. Journal of Power
Sources, 184(1): 44-51.
29.
Kamjornsupamitr,
T., Sangthumchai, T., Saejueng, P., Sumranjit, J., Hunt, A. J. and Budsombat,
S. (2021). Composite proton conducting membranes from chitosan, poly(vinyl
alcohol) and sulfonic acid-functionalized silica nanoparticles. International
Journal of Hydrogen Energy, 46(2):
2479-2490.