Malaysian
Journal of Analytical Sciences Vol 26 No 3
(2022): 581 - 588
PHOTOCATALYTIC HYDROGEN GENERATION FROM WATER BY TiO2/Co3O4
COMPOSITE PHOTOCATALYSIS
(Penjanaan Hidrogen Fotokatalitik dari
Molekul Air Mengunakan Komposi Fotomangkin TiO2/Co3O4)
Siti Nurul Falaein Moridon1, Dian Anggraini2, Khuzaimah Arifin1*, Lorna Jeffery Minggu1, Mohammad B. Kassim1,3
1Fuel Cell
Institute,
Universiti Kebangsaan Malaysia, 43600 UKM
Bangi, Selangor, Malaysia
2Department
of Chemistry, Faculty of Mathematic and Natural Science,
Universitas
Riau,Kampus Binawidya, Km 12.5 Simpang Baru, Pekan baru, Riau, Indonesia,
3Faculty
of Science and Technology,
Universiti
Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
*Corresponding
author: khuzaim@ukm.edu.my
Received: 28 November 2021; Accepted: 6 March 2022;
Published: 27 June 2022
Abstract
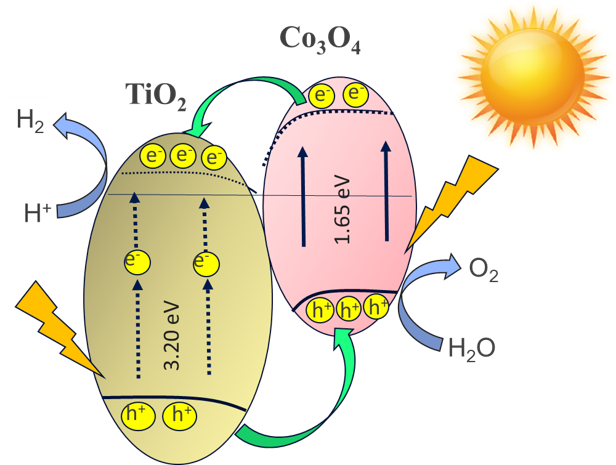
Titanium dioxide (TiO2)
is one of the most studied materials as photocatalyst of water splitting for
hydrogen generation. However, TiO2 has a large band gap of around
3.2 eV, limits its absorption energy to visible light, and the photoexcitation
products, such as electron and hole, are recombined rapidly. One method to overcome
this problem is by creating a composite heterojunction with other semiconductor
materials. In this study, the photocatalytic hydrogen generation of water
splitting by TiO2/Co3O4 composite
photocatalyst was evaluated. The composite was prepared through hydrothermal
synthesis assisted by ball mill crushing, and the powder was annealed at 550 °C. The percentage of
Co3O4 loading on the TiO2 varied at 0.5% w/w (TC-05),
1% w/w (TC-1), and 2% w/w (TC-2) to study the suitable amount of Co3O4.
The surface morphology of the composites was investigated through field
emission scanning electron microscopy (FESEM) analyses. Results showed that
nanosphere and cubic–shaped morphologies were obtained. For the hydrogen
performance analysis, two different conditions of photocatalytic hydrogen
generation, which are in pure water and water with addition of 10 vol% of
methanol solution as the sacrificial reagent, were measured by using a hydrogen
sensor (UNISENSE). TC-1 showed the highest hydrogen production in the pure water,
which is 6.75 µmol h1 g1 compared with others. The
addition of 10% methanol enhanced the hydrogen production by three times
compared with pure water (20.22 µmol h−1 g−1.
The superior heterojunction of TiO2 and Co3O4
performance can be used in practical applications to enhance the photocatalytic
properties of TiO2.
Keywords:
cobalt oxide,
hydrogen production, PEC, titanium dioxide
Abstrak
Titanium
dioksida (TiO2) adalah salah satu bahan yang paling banyak dikaji
sebagai fotomangkin pembelahan molekul air untuk penjanaan hidrogen. Walau
bagaimanapun, TiO2 mempunyai jurang jalur yang besar sekitar 3.2 eV,
yang mengehadkan tenaga penyerapannya kepada cahaya nampak, dan hasil
fotopengujaan, iaitu, elektron dan lubang mengakibatkan bergabung semula dengan
cepat. Salah satu cara untuk mengatasi masalah tersebut ialah dengan mencipta
gabungan komposit dengan bahan semikonduktor lain. Dalam kajian ini, untuk
penjanaan hidrogen bagi pembelahan molekul air fotomangkin gabungan komposit
TiO2/Co3O4 telah dinilai. Kaedah sintesis
hidroterma dibantu oleh proses penghancuran penggilingan bebola yang digunakan
untuk menyediakan komposit berikutan penyepuhlindapan serbuk pada 550 °C. Peratusan
pengabungan Co3O4 pada TiO2 telah divariasi
pada 0.5% w/w (TC-05), 1% w/w (TC-1), dan 2% w/w (TC-2) untuk mengkaji kadar Co3O4
yang sesuai. Morfologi permukaan komposit telah disiasat menggunakan analisis
FESEM. Berdasarkan FESEM menunjukkan nanosfera dan berbentuk kubik. Untuk
analisis prestasi hidrogen, dua keadaan berbeza penjanaan hidrogen
fotokatalitik iaitu dalam air tulen dan air ditambah 10 vol % larutan metanol
sebagai reagen, dan telah diukur menggunakan sensor hidrogen (UNISENSE).
Hasilnya, TC-1 menunjukkan pengeluaran hidrogen tertinggi dalam air tulen iaitu
6.75 µmol h-1 g-1 berbanding yang lain. Penambahan 10%
metanol meningkatkan pengeluaran hidrogen 3 kali lebih tinggi berbanding dengan
air tulen 20.22 µmol h-1 g-1. Gabungan komposit TiO2
dan Co3O4 boleh digunakan dalam aplikasi praktikal
meningkatkan sifat fotomangkin TiO2.
Kata kunci: kobalt oksida, pengeluaran hidrogen, PEC, titanium dioksida
Graphical Abstract
References
1. Acar, C. and Dincer,
I. (2020). The potential role of hydrogen as a sustainable transportation fuel
to combat global warming. International Journal of Hydrogen Energy, 45(5):
3396-3406.
2. Rath,
B. N., Akram, V., Bal, D. P. and Mahalik, M. K. (2019). Do fossil fuel and
renewable energy consumption affect total factor productivity growth? Evidence
from cross-country data with policy insights. Energy Policy, 127: 186-199.
3. Griffiths,
S., Sovacool, B. K., Kim, J., Bazilian, M. and Uratani, J. M. (2021).
Industrial decarbonization via hydrogen: A critical and systematic review of
developments, socio-technical systems and policy options. Energy Research
& Social Science, 80: 102208.
4. Zhao,
F., Mu, Z., Hao, H., Liu, Z., He, X., Victor Przesmitzki, S. and Ahmad Amer, A.
(2020). Hydrogen fuel cell vehicle development in China: An industry chain
perspective. Energy Technology, 8(11): 2000179.
5. Hosseini,
S. E. and Wahid, M. A. (2020). Hydrogen from solar energy, a clean energy
carrier from a sustainable source of energy. International Journal of Energy
Research, 44(6): 4110-4131.
6. Mah,
A. X. Y., Ho, W. S., Bong, C. P. C., Hassim, M. H., Liew, P. Y., Asli, U. A.,
... and Chemmangattuvalappil, N. G. (2019). Review of hydrogen economy in
Malaysia and its way forward. International Journal of Hydrogen Energy, 44(12):
5661-5675.
7. Ahmad,
H., Kamarudin, S. K., Minggu, L. J. and Kassim, M. (2015). Hydrogen from
photo-catalytic water splitting process: A review. Renewable and Sustainable
Energy Reviews, 43: 99-610.
8. Acar,
C. and Dincer, I. (2019). Review and evaluation of hydrogen production options
for better environment. Journal of Cleaner Production, 218: 835-849.
9. Qureshy,
A. M., Ahmed, M. and Dincer, I. (2019). Performance assessment study of
photo-electro-chemical water-splitting reactor designs for hydrogen production.
International Journal of Hydrogen Energy, 44(18): 9237-9247.
10. Arifin,
K., Yunus, R. M., Minggu, L. J. and Kassim, M. B. (2021). Improvement of TiO2
nanotubes for photoelectrochemical water splitting. International Journal of
Hydrogen Energy, 46(7): 4998-5024.
11. Moridon,
S. N. F., Salehmin, M. I., Mohamed, M. A., Arifin, K., Minggu, L. J. and Kassim,
M. B. (2019). Cobalt oxide as photocatalyst for water splitting:
Temperature-dependent phase structures. International Journal of Hydrogen
Energy, 44(47): 25495-25504.
12. Anggraini,
D., Wardani, P. K., Agustina, M., Awaluddin, A.and Arifin, K. (2019). TiO2/Co3O4
Composite as photoanode of photoelectrochemical water splitting. In Journal
of Physics: Conference Series, 1351: 012032.
13. Moridon,
S. N. F., Salehmin, M. N. I., Arifin, K., Minggu, L. J. and Kassim, M. B.
(2021). Synthesis of cobalt oxide on FTO by hydrothermal method for
photoelectrochemical water splitting application. Applied Sciences, 11(7):
3031.
14. Kumar,
S.G. and Rao, K. K. (2017). Comparison of modification strategies towards
enhanced charge carrier separation and photocatalytic degradation activity of
metal oxide semiconductors (TiO2, WO3 and ZnO), Applied Surface Science, 2017: 391124-148.
15. Alenzi,
N., Liao, W. S., Cremer, P. S., Sanchez-Torres, V., Wood, T. K.,
Ehlig-Economides, C. and Cheng, Z. (2010). Photoelectrochemical hydrogen
production from water/methanol decomposition using Ag/TiO2 nanocomposite
thin films. International Journal of Hydrogen Energy, 35(21):
11768-11775.
16. Wang,
L., Tang, G., Liu, S., Dong, H., Liu, Q., Sun, J. and Tang, H. (2022).
Interfacial active-site-rich 0D Co3O4/1D TiO2 pn
heterojunction for enhanced photocatalytic hydrogen evolution. Chemical
Engineering Journal, 428: 131338.