Malaysian
Journal of Analytical Sciences Vol 26 No 2
(2022): 283 - 294
CHROMIUM (VI) ANALYSIS IN EFFLUENTS USING LIQUID-LIQUID EXTRACTION
COUPLED WITH FLAME ATOMIC ABSORPTION SPECTROMETRY
(Analisis Kromium (VI) dalam
Efluen Menggunakan Pengekstrakan Cecair-Cecair Bersama Spektrometri Serapan
Nyalaan Atom)
Nguyen Cong-Hau1, Le-Thi Anh-Dao1, Nguyen Thanh-Nho1*, Le-Thi Huynh-Mai2, Le Nhon-Duc3, Do Minh-Huy1
1Faculty of Environmental and Food
Engineering,
Nguyen Tat Thanh
University, Ho Chi Minh City, Vietnam
2Faculty of Chemistry,
University of Science, Vietnam National
University Ho Chi Minh City, Vietnam
3Warrantek Joint Stock Company, Testing Center, Can Tho City,
Vietnam
*Corresponding
author: ntnho@ntt.edu.vn
Received: 1 September 2021;
Accepted: 10 January 2022;
Published: 28 April 2022
Abstract
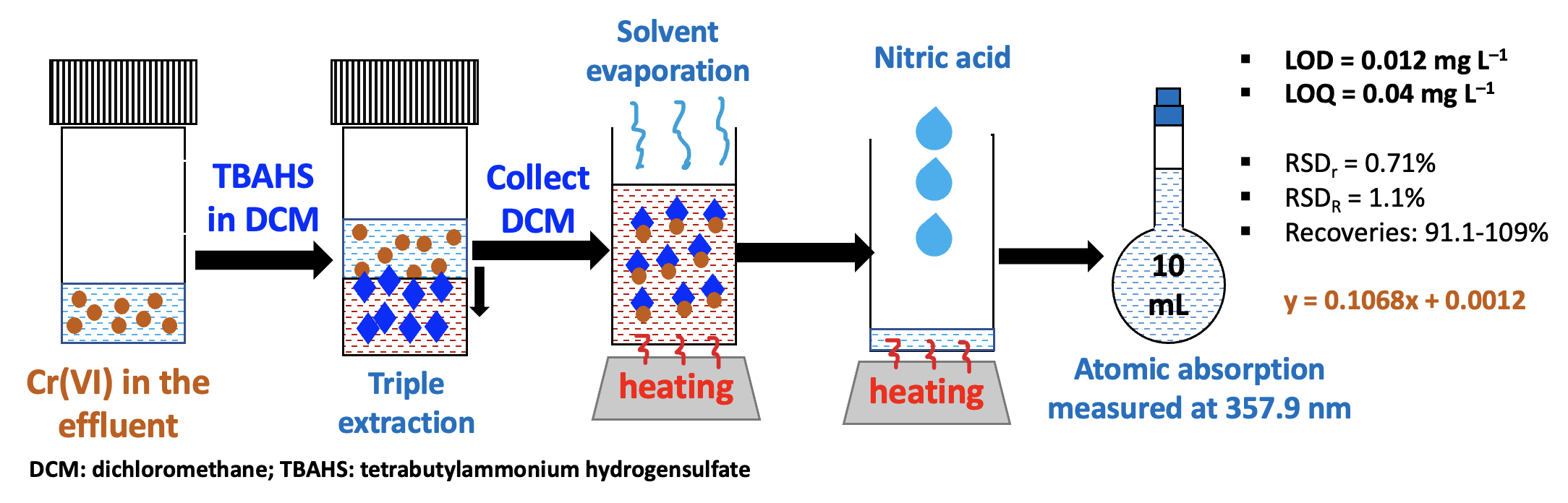
Simple sample preparation was investigated and
developed to selectively determine Cr(VI) in wastewater samples or effluents based on the liquid-liquid
extraction principle using
tetrabutylammonium hydrogensulfate (TBAHS) as the ion-pair reagent in an acidic
medium. TBAHS was prepared in an organic solvent to improve the extraction
efficiency. The extracted Cr(VI) in the organic phase endured the acid
digestion, and its atomic
absorption was measured at 357.9
nm. The influences of several working parameters, namely, organic solvents (methyl isobutyl
ketone-MIBK, dichloromethane-DCM, and chloroform), pH values (lower than 1.0, 1.0,
2.0, and 3.0) in the
aqueous phase, TBAHS concentrations in the organic solvent (0.02, 0.04, 0.05, and 0.06 mol L–1), extraction duration (from 3 to 30 minutes), number of extraction cycles (single or repeated extraction), sample preservation duration at ambient
temperature, and co-existence of Cr(III) in the sample matrices, were investigated to discover the optimized
working parameters. The results showed that dichloromethane (DCM)
was the most effective extraction solvent. The most favorable conditions for
complex formation were determined as follows: a pH of around 1.0 to 3.0; 0.05
mol L–1 TBAHS prepared in DCM, triple extraction, and a shaking duration of
15 minutes for each extraction cycle. The calibration curve was linear in the
range of 0.05, 0.10, 0.20, 0.40, 0.60, 0.90, 1.2, 1.5 and 2.0 mg L–1, and the regression equation
was y = 0.1068x + 0.0012 with R2 = 0.9994, exhibiting goodness of
linearity. The method detection and quantification limit values were estimated
to be 0.012 mg L–1 and 0.04 mg L–1, respectively. The
repeatability (RSDr = 0.71%) and reproducibility (RSDR =
1.1%) were favorable according to
the requirements presented in Appendix F of AOAC (2016) for analytical method
validation. The proposed method was applied to real wastewater samples and
spiked samples, showing very low Cr(VI) concentrations for most samples and
proper recoveries (91.1-109%).
Keywords: Cr(VI), Cr(III), liquid-liquid extraction,
TBAHS, dichloromethane, DCM,
ion-pair reagent
Abstrak
Penyediaan
sampel yang mudah telah dikaji dan dibangunkan bagi penentuan terpilih
Cr(VI) di dalam air sisa atau efluen berdasarkan
prinsip pengekstrakan cecair-cecair menggunakan tetrabutylammonium
hidrogensulfat (TBAHS) sebagai reagen pasangan ion di dalam medium berasid.
TBAHS telah disediakan dalam pelarut organik bagi tujuan meningkatkan
keberkesanan pengekstrakan. Cr(VI) yang telah diekstrak di dalam fasa organik
melalui penghadaman asid, dan serapan atom telah diukur pada 357.9 nm. Pengaruh
parameter seperti pelarut organik (metil
isobutil keton-MIBK, diklorometana-DCM, dan klorofom), nilai pH (dibawah 1.0,
1.0, 2.0 dan 3.0) di dalam fasa akues, kepekatan TBAHS di dalam pelarut organik
(0.02,
0.04, 0.05, dan 0.06 mol L–1), tempoh
pengekstrakan (dari 3 hingga 30 minutes), bilangan kitaran pengekstrakan
(pengekstrakan tunggal atau ulangan), tempoh pengawetan sampel pada suhu
sekitar, dan kehadiran bersama Cr(III) dalam matrik sampel turut dikaji untuk
penentuan parameter kerja yang optimum. Hasil kajian menunjukkan diklorometana
(DCM) paling efektif sebagai pelarut pengekstrakan. Keadaan paling baik untuk
penghasilan kompleks ditentukan seperti berikut: pH antara 1.0 hingga 3.0; 0.05 mol L–1 TBAHS disediakan dalam DCM, tiga kali
pengekstrakan, dan masa goncangan ialah 15 minit bagi setiap kitaran
pengekstrakan. Lengkung kalibrasi adalah linear pada julat 0.05 hingga 2.0 mg L–1, dan
persamaan regresi ialah y = 0.1068x + 0.0012 dan R2 = 0.9994. Had
pengesanan dan kuantifikasi telah dihitung masing-masing pada 0.012 mg L–1 and 0.04 mg L–1.
Kebolehulangan (RSDr = 0.71%) dan kebolehhasilan semula (RSDR
= 1.1%) adalah baik berdasarkan keperluan yang dinyatakan dalam Appendix F of
AOAC (2016) bagi validasi kaedah analisis. Kaedah yang dicadang ini telah
digunapakai bagi analisis sampel air sisa sebenar dan sampel yang dipaku, ia
menunjukkan kepekatan Cr(VI) yang rendah dan perolehan semula yang baik (91.1-109%).
Kata
kunci: Cr(VI), Cr(III),
pengekstrakan cecair-cecair, diklorometana, reagen pasangan ion
Graphical Abstract
References
1. Ali, H., Khan, E. and Ilahi, I. (2019). Environmental
chemistry and ecotoxicology of hazardous heavy metals: environmental
persistence, toxicity, and bioaccumulation. Journal
of Chemistry, 2019 (6730305): 1-14.
2. Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. and Sutton, D.J. (2012). Heavy metal toxicity and
the environment. Springer.
3. Saha, R., Nandi, R. and Saha, B. (2011). Sources and
toxicity of hexavalent chromium. Journal
of Coordination Chemistry, 64(10): 1782-1806.
4. Oliveira,
H. (2012). Chromium as an environmental pollutant: Insights on induced plant
toxicity. Journal of Botany, 2012(375843):
1-8.
5. Jia, X., Gong, D., Xu, B., Chi, Q. and Zhang, X.
(2016). Development of a novel, fast, sensitive method for chromium speciation
in wastewater based on an organic polymer as solid phase extraction material
combined with HPLC–ICP-MS. Talanta,
147: 155-161.
6. Séby, F. and Vacchina, V. (2018). Critical assessment
of hexavalent chromium species from different solid environmental, industrial
and food matrices. Trends in Analytical
Chemistry, 104: 54-68.
7. Ouejhani,
A., Dachraoui,
M.,
Lalleve, G. and Fauvarque, J.-F.
(2003). Hexavalent chromium recovery by
liquid-liquid extraction with tributylphosphate from acidic chloride media. Analytical
Sciences, 19(11): 1499-1504.
8. Kalidhasan, S. and Rajesh, N. (2009). Simple and
selective extraction process for chromium (VI) in industrial wastewater. Journal of Hazardous Materials,
170(2-3): 1079-1085.
9. Noro, J., Maruyama, K. and Komatsu, Y. (2002).
Separation of chromium (III) and chromium (VI) by the combination of solvent
and ion exchange methods. Analytical
Sciences/Supplements Proceedings of IUPAC International Congress on Analytical
Sciences, 2001: 1333-1336.
10. Islam, F. and Biswas, R. (1979). The solvent
extraction of chromium (III) with bis-(2-ethyl hexyl) phosphoric acid in
benzene and other solvents. Journal of
Inorganic and Nuclear Chemistry, 41(2): 229-233.
11. Gardner, M. and Comber, S. (2002). Determination of
trace concentrations of hexavalent chromium. Analyst, 127(1): 153-156.
12. Shinde, V. and Khopkar, S. (1970). Extraction of
chromium (VI) with 4-methyl-3-pentene-2-one and subsequent photometric
determination as diphenylcarbazide complex. Fresenius'
Zeitschrift für analytische Chemie, 249(4): 239-241.
13. Venkateswaran, P. and Palanivelu, K. (2004). Solvent
extraction of hexavalent chromium with tetrabutyl ammonium bromide from aqueous
solution. Separation and Purification
Technology, 40(3): 279-284.
14. Konieczka, P. and Namiesnik, J. (2016). Quality
assurance and quality control in the analytical chemical laboratory: A
practical approach, CRC Press.
15. Ellison, S. L., Barwick, V. J. and Farrant, T. J. D.
(2009). Practical statistics for the analytical scientist: A bench guide, Royal
Society of Chemistry.
16. ISO 5667-3:2003 (2003). Water
quality-Sampling-Part 3: Guidance on the preservation and handling of water
samples.
17. ISO 5667-10:2020 (2020). Water
quality-Sampling-Part 10: Guidance on sampling of waste waters.
18. Wypych,
G. (2001). Handbook of solvents, ChemTec Publishing.
19. Polarity Index. https://macro.lsu.edu/howto/
solvents/polarity%20index.htm. [Assess online 20 December 2020].
20. Kalidhasan, S., Ganesh, M., Sricharan, S. and Rajesh,
N. (2009). Extractive separation and determination of chromium in tannery
effluents and electroplating waste water using tribenzylamine as the
extractant. Journal of Hazardous
Materials, 165(1-3): 886-892.
21. Baig, J. A., Kazi, T. G., Elci, L., Afridi, H. I., Khan, M. I. and Naseer, H. M. (2013). Ultratrace determination of Cr (VI) and Pb
(II) by microsample injection system flame atomic spectroscopy in drinking
water and treated and untreated industrial effluents. Journal of Analytical Methods in Chemistry, 2013: 629495
22. Sereshti,
Khojeh, V. and
Samadi, S. (2011). Optimization
of dispersive liquid–liquid microextraction coupled with inductively coupled
plasma-optical emission spectrometry with the aid of experimental design for
simultaneous determination of heavy metals in natural waters. Talanta, 83(3): 885-890.
23. Tandon, R., Crisp, P., Ellis, J. and Baker, R. (1984).
Effect of pH on chromium (VI) species in solution. Talanta, 31(3): 227-228.
24. Kalidhasan, S. and Rajesh, N. (2009). Simple and
selective extraction process for chromium (VI) in industrial wastewater. Journal of Hazardous Materials,
170(2-3): 1079-1085.
25. Sperling, M., Xu, S. and Welz, B. (1992).
Determination of chromium (III) and chromium (VI) in water using flow injection
on-line preconcentration with selective adsorption on activated alumina and
flame atomic absorption spectrometric detection. Analytical Chemistry, 64(24): 3101-3108.
26. Palmer, C. D. (1994). Natural attenuation of hexavalent
chromium in ground water and soils: Superfund Technology Support Center for
Ground Water.
27. Leśniewska,
B., Jeglikowska,
A. and
Godlewska-Żyłkiewicz, B.
(2016). Chromium speciation in wastewater and
sewage by solid-phase extraction using a new diphenylcarbazone-incorporated
resin. Water, Air, & Soil Pollution, 227(8): 1-10.
28. Zhang,
L. and Lay, P. A. (1996). EPR Spectroscopic studies of the reactions of Cr (VI)
with l-ascorbic acid, l-dehydroascorbic acid, and 5,
6-O-isopropylidene-l-ascorbic acid in water. 1 Implications for chromium (VI)
genotoxicity. Journal of the American
Chemical Society, 118(50): 12624-12637.
29. Xu, X.-R., Li, H.-B., Li, X.-Y. and Gu, J.-D. (2004).
Reduction of hexavalent chromium by ascorbic acid in aqueous solutions.
Chemosphere, 57(7): 609-613.
30. QCVN 24:2009/BTNMT (2009).
National Technical Regulation on Industrial Wastewater.
31. Appendix F of AOAC
(2016). Guidelines for
Standard Method Performance Requirements.