Malaysian
Journal of Analytical Sciences Vol 26 No 2
(2022): 269 - 282
DIASTEREOSELECTIVE REDUCTION OF ENDOCYCLIC
β-ENAMINO ESTER: AN APPROACH TO PREPARE DIASTEREOPURE MULTISUBSTITUTED
PYRROLIDINE β-AMINO ESTERS
(Penurunan Diastereoselektif Terhadap Ester
β-Enamino: Sebuah Pendekatan untuk Menyediakan Ester β-Amino Pirolidin Multi-Terganti
Diastereotulen)
Ayisy Amirul Afti1*, Zurina Shaameri2,
Ahmad Sazali Hamzah2, Nor Saliyana Jumali1
1Department of Chemistry,
Kulliyyah of Science, International Islamic
University Malaysia, 25200 Kuantan, Pahang, Malaysia.
2Organic Synthesis Research Laboratory,
Institute of Science,
Universiti Teknologi MARA, 42300 Bandar Puncak
Alam, Selangor, Malaysia.
*Corresponding author: ayisypale@gmail.com
Received: 26 November 2021;
Accepted: 27 February 2022; Published: 28 April 2022
Abstract
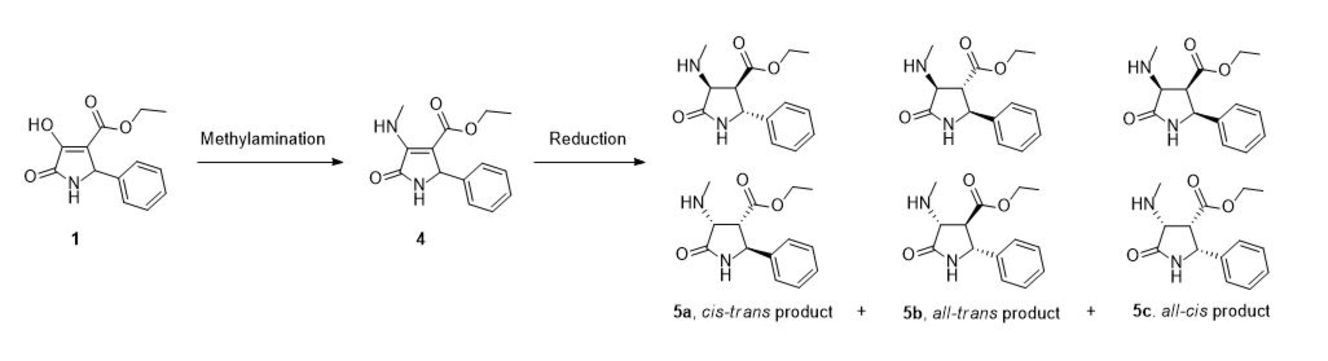
A set of chiral multisubstituted pyrrolidine diastereomers
containing β-amino ester moiety 5a-c were successfully synthesized
from
4-carbethoxy-2,3-dioxo-5-phenylpyrrolidine 1. The key
intermediate endocyclic β-enamino ester 4
were prepared by methylamination of the precursor 1. Afterwards,
acid-catalyzed reduction of 4 using sodium cyanoborohydride afforded
target products 5 with moderate diastereoselectivity. Other procedures
to reduce 4 including the use of sodium triacetoxyborohydride in acetic
acid and catalytic hydrogenations are compared with the aforementioned approach
in terms of selectivity. We hereby describe several plausible reaction mechanisms from the outcome of this
experiment.
Keywords: pyrrolidine, β-enamino ester,
diastereoselective reduction, patalytic hydrogenation, sodium cyanoborohydride
Abstrak
Satu set diastereomer pirolidin multi-terganti kiral yang
mengandungi bahagian ester β-amino 5a-c telah berjaya disintesis
dari
4-karbetoksi-2,3-diokso-5-fenilpirolidin 1. Perantara utama,
ester β-enamino endosiklik 4, telah disediakan melalui metilaminasi
pendahulu 1. Kemudian, penurunan 4 yang dimangkinkan oleh asid
menggunakan natrium sianoborohidrid menghasilkan produk sasaran 5 dengan
diastereoselektiviti yang sederhana. Tatacara lain untuk menurunkan 4 termasuk penggunaan
natrium triasetoksiborohidrid dalam asid asetik dan penghidrogenan mangkin
telah dibandingkan dengan kaedah terdahulu dari sudut selektiviti. Di sini,
kami menghuraikan beberapa mekanisme tindakbalas yang munasabah daripada hasil
eksperimen ini.
Kata kunci: pirolidin, ester β-enamino, penurunan diastereoselektif,
penghidrogenan mangkin, natrium sianoborohidrid
Graphical Abstract
References
1.
Xin, D., &
Burgess, K. (2014). A Chemoselective Route to β-Enamino Esters and
Thioesters. Organic Letters, 16(8), 2108-2110.
2.
Southwick, P.
L., & Hofmann, G. H. (1963). Compounds in the pyrrolo[3,4-d]pyrimidine
series. Syntheses based on 2, 3-dioxopyrrolidines. The Journal of
Organic Chemistry, 28(5), 1332-1336.
3.
Madhav, R.,
Dufresne, R. F., & Southwick, P. L. (1973). The preparation of derivatives 9‐oxo‐2,3,4,9‐tetrahydro‐1H‐pyrrolo[3,4‐b]quinoline
and 7‐oxo‐7,9,10,11‐tetrahydro‐8H‐benzo[h]pyrrolo
[3,4‐b]quinoline. Journal of Heterocyclic Chemistry, 10(2),
225-228.
4.
Jia, Z.,
Nagano, T., Li, X., & Chan, A. S. (2013). Iodide‐Ion‐Catalyzed
Carbon–Carbon Bond‐Forming Cross‐Dehydrogenative Coupling for the
Synthesis of Indole Derivatives. European Journal of Organic Chemistry, 2013(5),
858-861.
5.
David, O.,
Fargeau-Bellassoued, M. C., & Lhommet, G. (2002). A short and convenient
synthesis of chiral heterocyclic β-enamino esters from halogeno acetylenic
esters. Tetrahedron Letters, 43(19), 3471-3474.
6.
Calvet, S.,
David, O., Vanucci-Bacqué, C., Fargeau-Bellassoued, M. C., & Lhommet, G.
(2003). Chiral heterocyclic β-enamino esters: convenient synthesis and
diastereoselective reduction. Tetrahedron, 59(33),
6333-6339.
7.
Hart, D. J.,
Hong, W. P., & Hsu, L. Y. (1987). Total synthesis of (±)-Lythrancepine II
and (±)-Lythrancepine III. The Journal of Organic Chemistry, 52(21),
4665-4673.
8.
Deutsch, H. M.,
Ye, X., Shi, Q., Liu, Z., & Schweri, M. M. (2001). Synthesis and
pharmacology of site specific cocaine abuse treatment agents: a new synthetic
methodology for methylphenidate analogs based on the Blaise reaction. European
Journal of Medicinal Chemistry, 36(4), 303-311.
9.
Calvet-Vitale,
S., Vanucci-Bacqué, C., Fargeau-Bellassoued, M. C., & Lhommet, G. (2005).
Stereocontrolled reduction of chiral pyrrolidine and piperidine β-enamino
esters: formal enantioselective synthesis of (+)-calvine. Tetrahedron, 61(32),
7774-7782.
10.
David, O.,
Blot, J., Bellec, C., Fargeau-Bellassoued, M. C., Haviari, G., Célérier, J. P.,
& Gardette, D. (1999). Enamino ester reduction: A short enantioselective
route to pyrrolizidine and indolizidine alkaloids. Synthesis of
(+)-laburnine,(+)-tashiromine, and (−)-isoretronecanol. The
Journal of Organic Chemistry, 64(9), 3122-3131.
11.
Haviari, G.,
Célérier, J. P., Petit, H., & Lhommet, G. (1993). Asymmetric synthesis with
chiral hydrogenolysable amines. A short synthesis of (-)-isoretronecanol. Tetrahedron
letters, 34(10), 1599-1600.
12.
Zhang, X., Mi,
N., Liu, H., & Liu, Y. (2019). An approach to highly efficient reduction of
β-enamino esters: A convenient synthesis of β-amino esters. Journal
of Chemical Research, 43(5-6), 196-200.
13.
Cimarelli, C.,
& Palmieri, G. (1996). Stereoselective reduction of enantiopure
β-enamino esters by hydride: a convenient synthesis of both enantiopure
β-amino esters. The Journal of Organic Chemistry, 61(16),
5557-5563.
14.
Furukawa, M.,
Okawara, T., Noguchi, Y., & Terawaki, Y. (1979). Asymmetric syntheses of
β-amino acids by the reduction of enamines. Chemical and
Pharmaceutical Bulletin, 27(9), 2223-2226.
15.
Metten, B.,
Kostermans, M., Van Baelen, G., Smet, M., & Dehaen, W. (2006). Synthesis of
5-aryl-2-oxopyrrole derivatives as synthons for highly substituted
pyrroles. Tetrahedron, 62(25), 6018-6028.
16.
Mohammat, M.
F., Najim, N., Mansor, N. S., Sarman, S., Shaameri, Z., Zain, M. M., &
Hamzah, A. S. (2011). Synthesis and bioactivity of some
2-oxo-5-aryl-3-hydrazone and 2-oxo-5-aryl-4-hydrazone pyrrolidine
derivatives. ARKIVOC: Online Journal of Organic Chemistry.
17.
Mohammat, M.
F., Mansor, N. S., Shaameri, Z., & Hamzah, A. S. (2015). Diastereoselective
Reduction of 2,3-Dioxo-4-carboxy-5-substituted Pyrrolidines Using NaBH4/AcOH
and Heterogenous Hydrogenation Reactions. Journal of the Korean
Chemical Society, 59(1), 31-35.
18.
Rashid, F. N. A. A., Mohammat, M. F., Shaameri, Z., &
Hamzah, A. S. (2019). Synthesis of novel 3,4-fused pyrazolidinone γ-lactam
bicyclic moieties from 2,3-dioxo-4-carboxy-5-(substituted) pyrrolidines. Organic
Communications, 12(3), 121-131.
19.
Rashid, F. N.
A. A., Mohammat, M. F., Bouchamma, F. E., Shaameri, Z., & Hamzah, A. S.
(2020). Facile Reduction of β-Enamino Oxopyrrolidine Carboxylates Mediated
by Heterogeneous Palladium Catalyst. Russian Journal of Organic
Chemistry, 56(6), 1082-1088.
20.
Wang, G. T.,
Chen, Y., Wang, S., Gentles, R., Sowin, T., Kati, W., ... & Kempf, D.
(2001). Design, synthesis, and structural analysis of influenza neuraminidase
inhibitors containing pyrrolidine cores. Journal of medicinal chemistry, 44(8),
1192-1201.
21.
Borch, R. F.,
Bernstein, M. D., & Durst, H. D. (1971). Cyanohydridoborate anion as a
selective reducing agent. Journal of the American Chemical Society, 93(12),
2897-2904.