Malaysian
Journal of Analytical Sciences Vol 26 No 2
(2022): 318 - 333
THE
PREPARATION AND APPLICATION OF ZINC SULFIDE AS PHOTOCATALYST FOR WATER
REMEDIATION: A MINI REVIEW
(Penyediaan
dan Aplikasi Zink Sulfida sebagai Pemangkin Cahaya untuk Rawatan Air: Ulasan
Ringkas)
Kavirajaa Pandian Sambasevam, Jamilin Rashida Adnan, Izyan
Najwa Mohd Norsham, Siti Nor Atika Baharin*
Advanced Material for Environmental Remediation (AMER)
Research Group,
Faculty of Applied Sciences,
Universiti Teknologi MARA, Cawangan Negeri Sembilan,
Kampus Kuala Pilah, 72000 Kuala Pilah, Negeri Sembilan, Malaysia
*Corresponding author: atikabaharin@uitm.edu.my
Received:
2 September 2021; Accepted: 7 March 2022; Published: 28 April 2022
Abstract
ZnS has gained attention as an effective photocatalyst
for the photocatalytic degradation method in wastewater treatment.
Photocatalysis is believed to be a promising solution to solve the problem of
water pollution and remove organic pollutants. Apart from other photocatalysts
such as ZnO, TiO2 and MoS2, ZnS is a developing
photocatalyst in this degradation method due to its large bandgap energy. This
review paper comprehensively considered the preparation (hydrothermal,
solvothermal, low temperature, green synthesis, solid-state reaction, and
microwave-assisted synthesis) of ZnS, application, and some challenges that
have been faced by photocatalytic degradation methods. The adsorption and
photocatalytic properties of ZnS depend on the different morphology and size
formed by different methods. ZnS modification presents higher decomposition
efficiency in removing organic pollutants.
Keywords:
metal
disulfide, organic pollutants, photocatalytic degradation, sustainable water
management
Abstrak
ZnS mendapat perhatian sebagai pemangkin
cahaya yang terbaik untuk melakukan rawatan terhadap air yang tercemar.
Fotokatalisis dipercayai sebagai penyelesaian dalam menyelesaikan masalah air
yang tercemar dan menyingkirkan pencemaran semulajadi yang terdapat di dalam
air. Selain daripada pemangkin cahaya seperti ZnO, TiO2 dan MoS2,
ZnS dijadikan sebagai pemangkin cahaya dalam kaedah pemulihan air kerana ZnS
mempunyai tenaga jurang pita yang tinggi. Kertas kajian ini merangkumi cara
penyediaan (hidroterma, solvoterma, teknik suhu rendah, sintesis hijau, tindak
balas keadaan pepejal, dan sintesis berteraskan gelombang mikro) ZnS, aplikasi
dan beberapa cabaran yang perlu di hadapi dalam proses rawatan air. Ciri-ciri
penyerapan dan fotokatalitik ZnS bergantung kepada perbezaan struktur permukaan
dan saiz yang terbentuk dari perbezaan penyediaan. Pengubahsuaian ZnS menunjukkan
kecekapan penguraian yang tinggi kepada pencemaran semulajadi.
Kata kunci: logam
disulfida, pencemar organik, penyingkiran fotokatalitik, pengurusan lestari air
Graphical Abstract
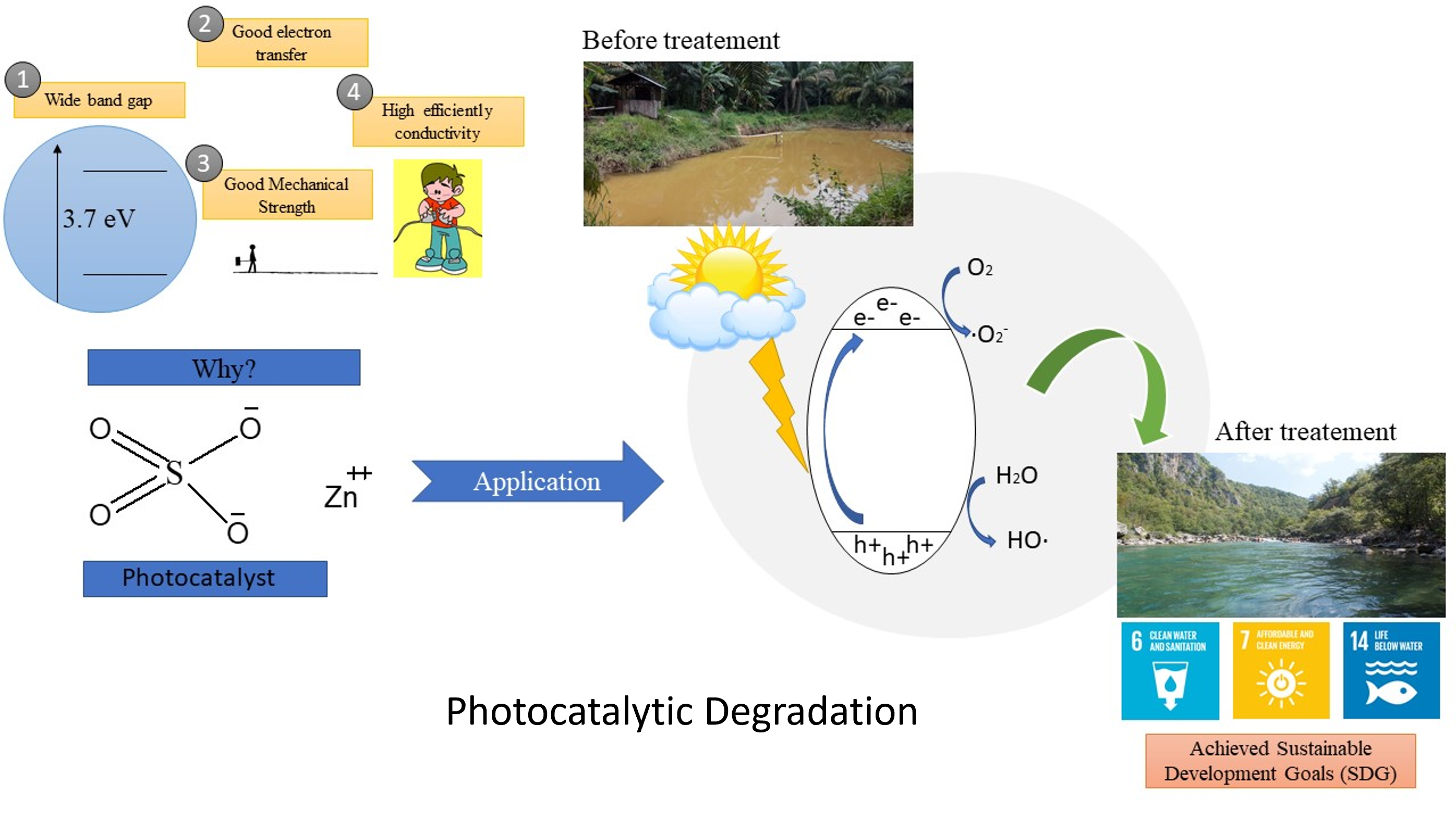
References
1.
Dehghanifard, E., Jafari, A. J., Kalantary, R. R., Mahvi, A. H.,
Faramarzi, M. A. and Esrafili, A. (2013). Biodegradation of 2, 4-dinitrophenol
with laccase immobilized on nano-porous silica beads. Iranian Journal of Environmental
Health Science and Engineering, 10(1): 25.
2.
Anjum, M., Oves, M., Kumar, R. and Barakat, M. A. (2017).
Fabrication of ZnO-ZnS@ polyaniline nanohybrid for enhanced photocatalytic
degradation of 2-chlorophenol and microbial contaminants in wastewater. International
Biodeterioration and Biodegradation, 119: 66-77.
3.
Ayodhya, D. and Veerabhadram, G. (2018). A review on recent
advances in photodegradation of dyes using doped and heterojunction based
semiconductor metal sulfide nanostructures for environmental protection. Materials
Today Energy, 9: 83-113.
4.
Salah, N., Hameed, A., Aslam, M., Babkair, S. S., and
Bahabri, F. S. (2016). Photocatalytic activity of V doped ZnO nanoparticles
thin films for the removal of 2-chlorophenol from the aquatic environment under
natural sunlight exposure. Journal of Environmental Management, 177: 53-64.
5.
Das, M. and Sarkar, D. (2017). One-pot synthesis of zinc
oxide-polyaniline nanocomposite for fabrication of efficient room temperature ammonia
gas sensor. Ceramics International, 43(14): 11123-11131.
6.
Allahyeran
and Mehrizad (2017). Comparison between different d-dimer cutoff values to
assess the individual risk of recurrent venous thromboembolism: Analysis of
results obtained in the DULCIS study. International Journal of Laboratory
Hematology 38(1): 42-49.
7.
Lai, K.,
Wei, W., Yingtao, Z., Meng, G., Ying, D. and Baibiao, H. (2012). Effects of oxygen
vacancy and n-doping on the electronic and photocatalytic properties of Bi2MO6
(M=Mo, W). Journal of Solid State Chemistry 187:103-108.
8.
Goswami, M., Sahoo, S., Meikap, A. K. and Ghosh, R. (2011).
Characterization, optical and dc electrical properties of polyaniline-zinc
sulphide nanocomposite. In International Conference on Nanoscience,
Engineering and Technology, 2011: 314-318.
9.
Bora, L. V. and Mewada, R. K. (2017). Visible/solar light
active photocatalysts for organic effluent treatment: Fundamentals, mechanisms
and parametric review. Renewable and Sustainable Energy Reviews, 76:
1393-1421.
10.
Byrne, C., Subramanian, G. and Pillai, S. C. (2018). Recent
advances in photocatalysis for environmental applications. Journal of
Environmental Chemical Engineering, 6(3): 3531-3555.
11.
Al-Hamdi,
Abdullah, M., Uwe, R. and Mika, S. (2017). Tin dioxide as a photocatalyst for
water treatment: A review. Process Safety and Environmental Protection
107:190-205.
12.
Mahvelati-Shamsabadi, T. and E. K. Goharshadi. (2017).
Photostability and visible-light-driven photoactivity enhancement of
hierarchical ZnS nanoparticles: The role of embedment of stable defect sites on
the catalyst surface with the assistant of ultrasonic waves. Ultrasonics Sonochemistry,
34: 78-89.
13.
Umar, M. and Aziz, H. A. (2013). Photocatalytic degradation
of organic pollutants in water. Organic Pollutants-Monitoring, Risk and
Treatment, 8: 196-197.
14. Munawaroh, H., Sari,
P. L., Wahyuningsih, S. and Ramelan, A.
H. (2018) The photocatalytic degradation of methylene blue using graphene oxide
(GO)/ZnO nanodrums. In AIP Conference Proceedings, 2014: p. 020119.
15.
Majhi, M., Choudhary, R. B., and Maji, P. (2017). HCl
protonated polymeric PANI-ZnS nanocomposites and measurement of their robust
dielectric, optical and thermal performance. Optik, 136: 181-191.
16.
Varanda, L. C., de Souza, C. G. S., Perecin, C. J., de
Moraes, D. A., de Queir z, D. F., Neves, H. R. and da Silva, T. L. (2019).
Inorganic and organic inorganic composite nanoparticles with potential
biomedical applications: Synthesis challenges for enhanced performance. In Materials
for Biomedical Engineering: pp. 47-99.
17.
Salavati-Niasari,
M., Fatemeh, D. and Mehdi, M. (2009). Synthesis and characterization of ZnS
nanoclusters via hydrothermal processing from [Bis(Salicylidene)Zinc(II)]. Journal
of Alloys and Compounds 470(1 2): 502-506.
18.
Hu, L.,
Feiyan, C., Pengfei, H., Lianpei, Z. and Xing, H. (2016). Hydrothermal
synthesis of SnO2/ZnS nanocomposite as a photocatalyst for degradation of
rhodamine b under simulated and natural sunlight. Journal of Molecular
Catalysis A: Chemical, 411: 203-213.
19.
Lee, G. J. and Wu, J. J. (2017). Recent developments in ZnS
photocatalysts from synthesis to photocatalytic applications -A review.
Powder Technology, 318: 8-22.
20.
Shakouri-Arani, M. and Salavati-Niasari, M. (2014). Synthesis
and characterization of wurtzite ZnS nanoplates through simple solvothermal
method with a novel approach. Journal of Industrial and Engineering
Chemistry, 20(5): 3179-3185.
21. Song, L., Zhang, S., Chen, B., Ge, J. and Jia, X. (2010).
Fabrication of ternary zinc cadmium sulfide photocatalysts with highly visible-light
photocatalytic activity. Catalysis Communications, 11(5): 387-390.
22.
Shahid, R., Toprak, M., Soliman, H. and Muhammed, M. (2012).
Low temperature synthesis of cubic phase zinc sulfide quantum dots. Open
Chemistry, 10(1): 54-58.
23. Guo, J., Khan, S., Cho, S. H., and Kim, J. (2019).
Preparation and immobilization of zinc sulfide (ZnS) nanoparticles on
polyvinylidene fluoride pellets for photocatalytic degradation of methylene
blue in wastewater. Applied Surface Science, 473: 425-432.
24.
Senapati,
U. S., Jha, D. K. and Sarkar D. (2013). Green synthesis and characterization of
ZnS nanoparticles. Research Journal of Physical Sciences,1(7):
2320-4796.
25.
Hudlikar,
M., Shreeram, J., Mayur, D. and Kisan, K. (2012). Latex-mediated synthesis of
ZnS nanoparticles: Green synthesis approach. Journal of Nanoparticle Research,
14(5): 865.
26.
Kannan, S., Subiramaniyam, N. P. and Sathishkumar, M. (2020).
A novel green synthesis approach for improved photocatalytic activity and
antibacterial properties of zinc sulfide nanoparticles using plant extract of Acalypha
indica and Tridax procumbens. Journal of Materials Science: Materials in
Electronics, 31(12): 9846-9859.
27.
Lan, C.,
Kunquan, H., Wenzhong, W. and Guanghou, W. (2003). Synthesis of ZnS nanorods by
annealing precursor ZnS nanoparticles in NaCl flux. Solid State Communications,
125(9): 455-58.
28.
Jothibas, M., Manoharan, C., Jeyakumar, S. J., Praveen, P.,
Punithavathy, I. K. and Richard, J. P. (2018). Synthesis and enhanced
photocatalytic property of Ni doped ZnS nanoparticles. Solar Energy, 159:
434-443.
29.
Hu, H., Wang, X., Liu, F., Wang, J. and Xu, C. (2011). Rapid
microwave-assisted synthesis of graphene nanosheets zinc sulfide nanocomposites:
Optical and photocatalytic properties. Synthetic Metals, 161(5-6):
404-410.
30.
Boulkroune, R., Sebais, M., Messai, Y., Bourzami, R., Schmutz, M.,
Blanck, C., ... and Boudine, B. (2019). Hydrothermal synthesis of strontium-doped
ZnS nanoparticles: structural, electronic and photocatalytic
investigations. Bulletin of Materials Science, 42(5): 1-8.
31.
Chen, Y., Yin, R. H. and Wu, Q. S. (2012). Solvothermal synthesis of
well-disperse ZnS nanorods with efficient photocatalytic properties. Journal
of Nanomaterials, 2012: 560310.
32.
Suganya, S., Jothibas, M. and Jeyakumar, S. J. (2019). Solid state
synthesis of cadmium doped ZnS with excellent photocatalytic activity and enhanced
visible light emission. Journal of Materials Science: Materials in
Electronics, 30(8): 7916-7927.
33.
Abbasi, M., Rafique, U., Murtaza, G. and Ashraf, M. A. (2018).
Synthesis, characterisation and photocatalytic performance of ZnS coupled Ag2S
nanoparticles: A remediation model for environmental pollutants. Arabian
Journal of Chemistry, 11(6): 827-837.
34.
Wang, W., Lee, G. J., Wang, P., Qiao, Z., Liu, N. and Wu, J. J. (2020).
Microwave synthesis of metal-doped ZnS photocatalysts and applications on
degrading 4-chlorophenol using heterogeneous photocatalytic ozonation
process. Separation and Purification Technology, 237: 116469.