Malaysian
Journal of Analytical Sciences Vol 26 No 1
(2022): 70 - 83
SUPRAMOLECULAR ASSEMBLIES OF 1,2-DISUBSTITUTED CYCLOHEXANE AMIDE
LIGANDS AND THEIR COORDINATION POLYMER: SYNTHESIS, CHARACTERISATION, AND CRYSTAL STRUCTURE
(Himpunan Supramolekul Ligan
Amida Sikloheksana 1,2-Tertukar Ganti dan Polimer Koordinatannya: Sintesis,
Pencirian, dan Struktur Hablur)
Nur
Shuhaila Haryani Haris1, Nafisah Mansor1, Maisara Abdul
Kadir1,2*
1Faculty of Science and Marine Environment,
2Advanced Nano Materials Research Group,
Faculty of Science and Marine Environment,
Universiti Malaysia Terengganu, 21030 Kuala Nerus,
Terengganu, Malaysia
*Corresponding
author: maisara@umt.edu.my
Received: 13 September
2021; Accepted: 18 December 2021; Published:
25 February 2022
Abstract
Supramolecular interactions such as hydrogen
bonding,
Keywords: supramolecular, coordination polymer, cyclohexane, hydrogen bonding,
racemic
Abstrak
Interaksi supramolekul seperti ikatan hidrogen,
susunan
Kata kunci: supramolekul,
polimer koordinatan, sikloheksana, ikatan hidrogen, rasemik
Graphical Abstract
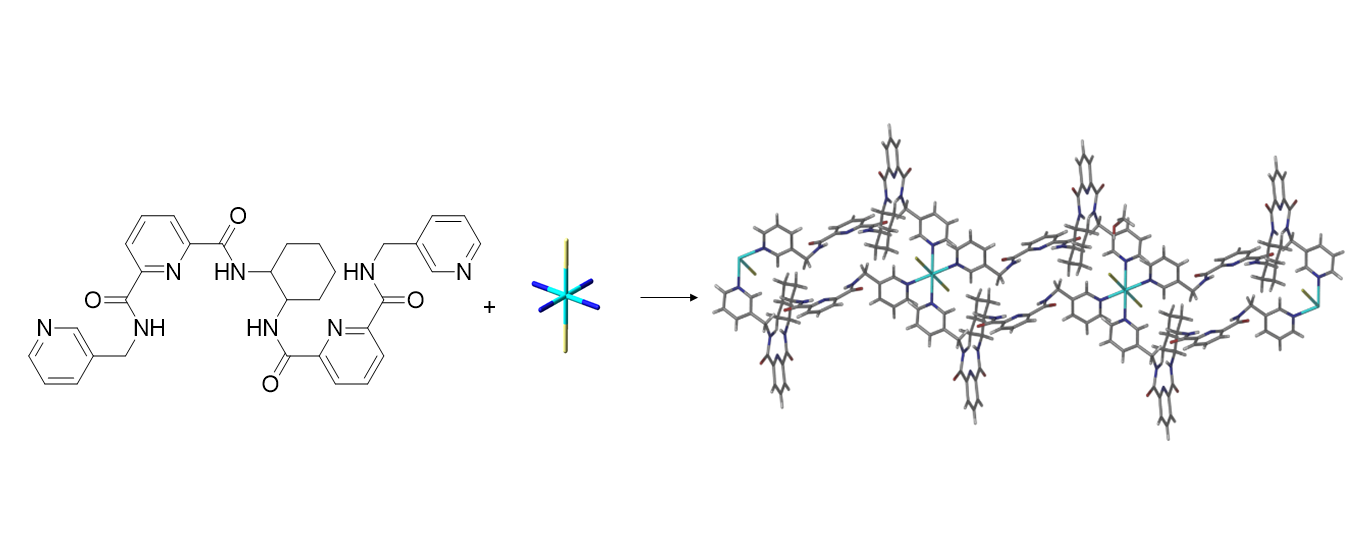
References
1. Lehn, J. M. (1988). Supramolecular chemistry-scope and perspectives
molecules, supermolecules, and molecular devices (nobel lecture). Angewandte Chemie International Edition in
English, 27(1): 89-112.
2. Lu, R., Zhang, X., Cheng, X., Zhang, Y., Zan, X. and Zhang, L. (2020).
Medical applications based on supramolecular self-assembled materials from
tannic acid. Frontiers in Chemistry,
8: 1-25.
3. Shuturminska, K., O'Malley, C., Collis, D. W. P., Conde, J. and Azevedo
H.S. (2018). Displaying biofunctionality on materials through template
self-assembly. Self-Assembling
Biomaterials, 341-370.
4. Qin, B., Yin, Z., Tang, X., Zhang, S., Wu, Y., Xu, J. F. and Zhang, X.
(2020). Supramolecular polymer chemistry: From structural control to functional
assembly. Progress in Polymer Science,
100: 101167.
5. Deng, J. H., Luo, J., Mao, Y. L., Lai, S., Gong, Y. N., Zhong, D. C. and
Lu, T. B. (2020).
6. Frey, P. A. (2004). Low barrier hydrogen bonds. Encyclopedia of Biological Chemistry, 2004: 594-598.
7. Wendler, K., Thar, J., Zahn, S. and Kirchner, B. (2010). Estimating the
hydrogen bond energy. The Journal of
Physical Chemistry A, 114: 9529-9536.
8. Castineiras, A., Fernandez-Hermida, N., Garcia-Santos, I.,
Gomez-Rodriguez, L., Gil, D. M. and Frontera, A. (2021). Supramolecular,
spectroscopic and computational analysis of weak interactions in some
thiosemicarbazones derived from 5-acetylbarbituric acid. Journal of Molecular Structure, 5: 131031.
9. Ghule, N. V., La, D. D., Bhosale, R. S., Kobaisi, M. A., Raynor, A. M.,
Bhosale, S. V. and Bhosale, S. V. (2016). Effect of amide hydrogen bonding
interaction on supramolecular self-assembly of naphthalene diimideamphiphiles
with aggregation induced emission. ChemistryOpen,
5(2): 157-163.
10. Rong, X., Lin, H., Liu, D., Wang, X., Liu, G. and Wang X. (2016).
Solvothermal synthesis, structures and properties of two new
octamolybdate-based compounds with tetrazole- and pyridyl-containing asymmetric
amide ligands. Inorganic Chemistry
Communications, 71: 9-14.
11. Haris, N. S. H., Mansor, N., Yusof, M. S. M., Sumby, C. J. and Kadir,
M.A. (2021). Investigating the potential of flexible and pre-organized
tetraamide ligands to encapsulate anions in one-dimensional coordination
polymers: synthesis, spectroscopic studies and crystal structures. Crystals, 11: 77.
12. Yusof, M. S. M., Ayob, N. A. C., Kadir, M. A. and Yamin, B. M. (2008).
1, 2-bis [N-(2, 2-dimethylpropionyl)
thioureido] cyclohexane. Acta
Crystallographica Section E: Structure Reports Online, 64(5): o937.
13. Shanmugharaj, A. M., Rhee, K. Y. and Ryu, S.H. (2006). Influence of
dispersing medium on grafting of aminopropyltriethoxysilane in swelling clay
materials. Journal of Colloid and
Interface Science, 298(2): 854 -859.
14. Oprea, S., Potolinca, V. O. and Varganici, C. D. (2016). Synthesis and
properties of polyurethane urea with pyridine-2,6-dicarboxamide moieties in
their structure. The Royal Society of
Chemistry, 6(108): 106904�106913.
15. Yalcin, S. P., Ceylan, U., Sarioglu, A. O. and Sonmez, M. (2015).
Sythesis, structural, spectral (FT-IR, 1H and 13C NMR and
UV-Vis), NBO and first order hyperpolarizability analysis of N-(4-nitrophenyl)-2,
2-dibenzoylacetamide by density functional theory. Journal of Molecular Structure, 1098: 400-407.
16. Basak, A., Ghosh, S. C., Das, A. K. and Bertolasi, V. (2005). A novel
azetidinyl
17. Kushwaha, N., Saini, R. K. and Kushwaha, S. K. S. (2011). Synthesis of
some amide derivatives and their biological activity. International Journal of ChemTech Research, 3(1): 203-209.
18. Ikawa, T., Nishiyama, T., Shigeta, T., Mohri, S., Morita, S.,
Takayanagi, S-I., Terauchi, Y., Morikawa, Y., Takagi, A., Ishikawa, Y., Fujii,
S., Kita, Y. and Akai, S. (2011). Ortho-selective
nucleophilic addition of primary aminesto silylbenzynes: synthesis of
2-silylanilines. Angewandte Chemie,
123: 5792-5795.
19. Hadadzadeh, H., Rezvani, A. R., Abdolmaleki, M. K., Ghasemi, K.,
Esfandiari, H. and Daryanavard, M. (2009). Pyridine-2,6-dicarboxylic acid
(dipic): crystal structure from co-crystal to a mixed ligand nickel(II)
complex. Journal of Chemical
Crystallography, 40(1): 48-57.
20. Kadir, M. A., Yusof, M. S. M., Sumby, C. J (2018). Conjoint experimental
and theoretical evaluation of zinc (II) coordination polymer as potential anion
receptors for nitrate and chromate. ASM
Journal of Science, 1: 136-146.