Malaysian
Journal of Analytical Sciences Vol 26 No 1
(2022): 39 - 46
MEASUREMENT OF SOLVENT
PROPERTIES USING KAMLET-TAFT APPROACH FOR APPLICATION IN SYNTHESIS
(Pengukuran Sifat Pelarut Menggunakan Pendekatan Kamlet-Taft untuk
Penggunaan dalam Sintesis)
Tariqul Islam1, A.
B. M. Helal Uddin1, Sahena Ferdosh2, Md. Zaidul Islam
Sarker1,3*
1Faculty of Pharmacy
2Faculty of Science
International Islamic University Malaysia, 25200 Kuantan,
Pahang, Malaysia
3Program Leader of Food Science Program, Cooperative Research, Education Extension Services,
Northern Marianas College, 501250, Saipan MP 96950, USA
*Corresponding author:
mdzaidul.sarker@marianas.edu
Received: 31 August 2021;
Accepted: 11 January 2022; Published: 25
February 2022
Abstract
Solvents are an unavoidable
part of pharmaceutical and chemical manufacturing/synthesis, most of them are
toxic or hazardous. The study on toxic solvent replacement is ongoing over the
world. Researchers are trying to overcome the hazardous issues that can be
possible using the mixture of hydrogen bond donor (HBD) and hydrogen bond
acceptor (HBA) solvent as a safe/recommended solvent mixture. This study
presents the possibility for the replacement/limitation of dipolar aprotic
solvent in drug synthesis using solvent-pair mixture where the Kamlet-Taft (KT)
parameter works as a tool to alternate the uses of such types of toxic
solvents. It has been simplified here among the many methods and equations of
the KT approach. The polarity (π*), basicity (β), and acidity
(α) of 10 pure solvents and 16 solvent-pair mixtures were measured
spectroscopically, utilizing well-suited dyes or indicators. The highest
absorption wavenumber value of indicators in the solution was selected and the
simplified KT equations were used to determine the solvent properties (π*,
β, α). Solvent mixtures were classified as per the solvent selection
guideline of GSK2016 and CHEM21. Four pure solvents (tetrahydrofuran,
dimethylformamide, dimethylsulfoxide, and acetone) exhibited low KT acidity,
high KT basicity, and high KT polarity. Eight aqueous solvent mixtures
(water-acetone, water-ethanol, water-isopropyl alcohol,
water-dimethylsulfoxide, water-dimethylformamide, water-tetrahydrofuran), and
two non-aqueous solvent mixtures (ethanol-dimethylformamide,
ethanol-dimethylsulfoxide) showed low KT acidity and high KT basicity. Solvent
classification by composite score showed that four solvent mixtures were as
recommended and 5 mixtures were near to recommended solvent among 16 solvent
mixtures. KT parameter was a simplified approach to determine which mixture can
bind with active pharmaceutical ingredients (API) that is indicated by KT
solvatochromic properties and solvent classification.
Keywords: Kamlet-Taft
parameters, hazardous solvent, solvent-pair mixture, dipolar aprotic solvent,
drug synthesis
Abstrak
Pelarut
adalah bahagian yang tidak dapat dielakkan dalam pembuatan/sintesis
farmaseutikal dan kimia, kebanyakannya beracun atau berbahaya. Kajian mengenai
penggantian pelarut toksik sedang dijalankan di seluruh dunia. Penyelidik
berusaha mengatasi masalah berbahaya yang mungkin dilakukan dengan menggunakan
campuran pelarut penderma ikatan hidrogen (HBD) dan pelarut ikatan hidrogen
(HBA) sebagai campuran pelarut yang selamat/disyorkan. Kajian ini menunjukkan
kemungkinan penggantian/pembatasan pelarut aprotik dipolar dalam sintesis ubat
dengan campuran pasangan pelarut di mana parameter Kamlet-Taft (KT) berfungsi
sebagai alat untuk mengganti penggunaan jenis pelarut toksik tersebut. Ini
telah dipermudahkan di sini antara banyak kaedah dan persamaan pendekatan KT.
Kekutuban (π*), asas (β), dan keasidan (α) daripada 10 pelarut
tulen dan 16 campuran pasangan pelarut telah diukur dengan menggunakan
spektroskopi, berdasarkan pewarna atau indikator yang sesuai. Nilai penyerapan
gelombang tertinggi dari indikator dalam larutan dipilih dan persamaan KT
digunakan untuk menentukan sifat pelarut (π*, β, α). Campuran
pelarut dikelaskan mengikut garis panduan pemilihan pelarut GSK 2016 dan CHEM21.
Empat pelarut tulen (tetrahidrofuran, dimetilformamida, dimetilsulfoksida, dan
aseton) menunjukkan keasidan KT rendah, asas KT tinggi, dan kekutuban KT
tinggi. Lapan campuran pelarut berasaskan air (air-aseton, air-etanol,
air-isopropil alkohol, air-dimetilsulfoksida, air-dimetilformamida,
air-tetrahidrofuran), dan dua campuran pelarut tidak berasaskan air
(etanol-dimetilformamida, etanol-dimetilsulfoksida) menunjukkan keasidan KT
rendah dan asas KT yang tinggi. Penggolongan terhadap 16 campuran pelarut berdasarkan
skor komposit menunjukkan empat campuran pelarut adalah seperti yang disyorkan
dan 5 campuran pelarut hampir dengan yang disyorkan. Parameter KT adalah
pendekatan yang dipermudah untuk menentukan campuran mana yang dapat mengikat
dengan bahan aktif farmaseutikal (API) yang ditunjukkan oleh sifat
solvatochromic KT dan klasifikasi pelarut.
Kata
kunci: parameter
Kamlet-Taft, pelarut berbahaya, campuran pasangan pelarut, pelarut aprotik
dipolar, sintesis ubat
Graphical Abstract
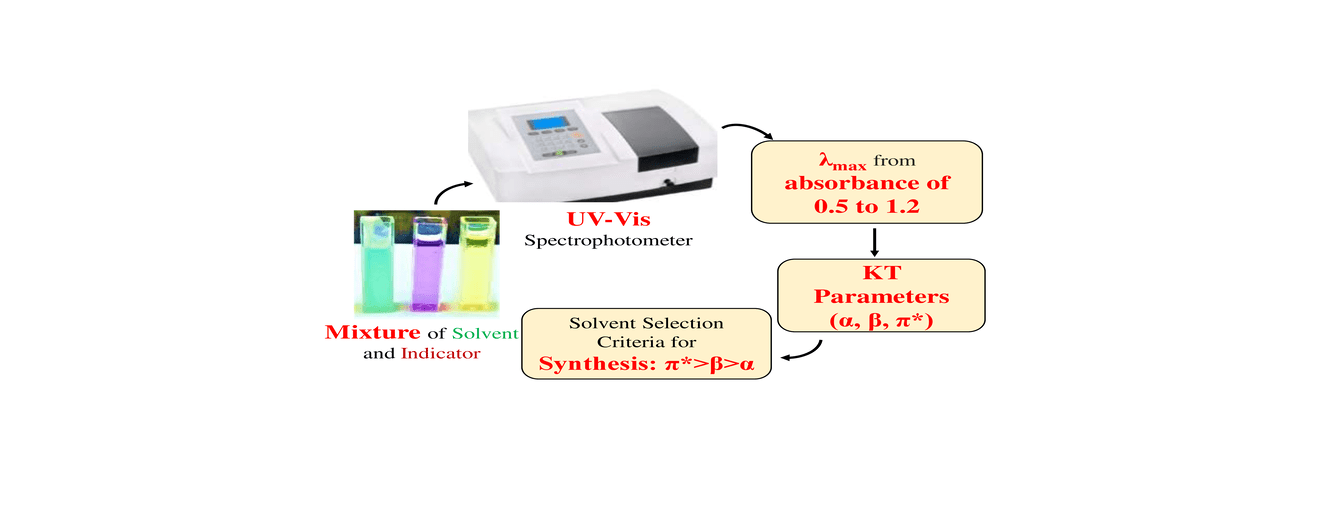
References
1. Kamlet, M. J., and Taft, R. W.
(1976). The solvatochromic comparison method. I. The beta-scale of solvent
hydrogen-bond acceptor (HBA) basicity. Journal of the American chemical
Society, 98(2): 377-383.
2. Labban, A. S. and Marcus, Y.
(1997). Solvatochromic parameters of ethanolamines. Journal of the Chemical
Society, Faraday Transactions, 93(1): 77-79.
3. Islam, T., Sarker, M. Z. I.,
Uddin, A. H., Yunus, K. B., Prasad, R., Mia, M. A. R. and Ferdosh, S. (2020).
Kamlet Taft parameters: A tool to alternate the usage of hazardous solvent in
pharmaceutical and chemical manufacturing/synthesis-A gateway towards green
technology. Analytical Chemistry Letters, 10 (5): 550-561.
4. Duereh, A., Guo, H., Honma,
T., Hiraga, Y., Sato, Y., Lee Smith Jr, R. and Inomata, H. (2018). Solvent
polarity of cyclic ketone (cyclopentanone, cyclohexanone): Alcohol (methanol,
ethanol) renewable mixed-solvent systems for applications in pharmaceutical and
chemical processing. Industrial & Engineering Chemistry Research,
57(22): 7331-7344.
5.
Duereh,
A., Sato, Y., Smith Jr, R. L., and Inomata, H. (2016). Analysis of the
cybotactic region of two renewable lactone water mixed-solvent systems that
exhibit synergistic Kamlet Taft basicity. The Journal of Physical Chemistry
B, 120(19): 4467-4481.
6.
Marcus,
Y. (1994). The use of chemical probes for the characterization of solvent
mixtures. Part 2. Aqueous mixtures. Journal of the Chemical Society, Perkin
Transactions 2(8): 1751-1758.
7.
Prat,
D., Wells, A., Hayler, J., Sneddon, H., McElroy, C. R., Abou-Shehada, S. and
Dunn, P. J. (2015). CHEM21 selection guide of classical-and less
classical-solvents. Green Chemistry, 18(1): 288-296.
8.
European
Medicines Agency (2019), ICH guideline Q3C (R6) on impurities: guideline for
residual solvents, step 5. https://www.ema.europa.eu/ en/ich-q3c-r6-residual-solvents
[Access online 08 October 2021].
9.
Byrne,
F. P., Jin, S., Paggiola, G., Petchey, T. H., Clark, J. H., Farmer, T. J.,
Hunt, A. J., McElroy, C. R. and Sherwood, J. (2016). Tools and techniques for
solvent selection: green solvent selection guides. Sustainable Chemical
Processes, 4(1): 1-24.
10.
Prat,
D., Hayler, J. and Wells, A. (2014). A survey of solvent selection guides. Green
Chemistry, 16(10): 4546-4551.
11.
Duereh,
A., Sato, Y., Smith Jr, R. L. and Inomata, H. (2017). Methodology for replacing
dipolar aprotic solvents used in API processing with safe hydrogen-bond donor
and acceptor solvent-pair mixtures. Organic Process Research &
Development, 21(1): 114-124.
12.
Duereh,
A., Sato, Y., Smith Jr, R. L. and Inomata, H. (2015). Spectroscopic analysis of
binary mixed-solvent-polyimide precursor systems with the preferential
solvation model for determining solute-centric Kamlet Taft solvatochromic
parameters. The Journal of Physical Chemistry B, 119(46): 14738-14749.
13.
Duereh,
A., Sato, Y., Smith Jr, R. L. and Inomata, H. (2015). Replacement of hazardous
chemicals used in engineering plastics with safe and renewable hydrogen-bond
donor and acceptor solvent-pair mixtures. ACS Sustainable Chemistry &
Engineering, 3(8): 1881-1889.
14.
Capello,
C., Fischer, U. and Hungerbhler, K. (2007). What is a green solvent? A
comprehensive framework for the environmental assessment of solvents. Green
Chemistry, 9(9): 927-934.
15.
Clark,
J. H., and Tavener, S. J. (2007). Alternative solvents: shades of green. Organic
Process Research & Development, 11(1): 149-155.
16.
Jessop,
P. G. (2011). Searching for green solvents. Green Chemistry, 13(6):
1391-1398.
17.
Ashcroft,
C. P., Dunn, P. J., Hayler, J. D. and Wells, A. S. (2015). Survey of solvent
usage in papers published in organic process research & development
1997-2012. Organic Process Research & Development, 19(7): 740-747.
18.
Sherwood, J., Granelli, J.,
McElroy, C. R. and Clark, J. H. (2019). A method of calculating the
Kamlet Abboud Taft solvatochromic parameters using COSMO-RS. Molecules, 24(12):
2209.
19.
Dolan, D. A., Sherman, D. A.,
Atkin, R., and Warr, G. G. (2016). Kamlet taft solvation parameters of solvate
ionic liquids. ChemPhysChem, 17(19): 3096-3101.
20.
Marcus,
Y. (1998). The properties of solvents. John Wiley & Sons, England: pp. 256
21.
Marcus,
Y. (1993). The properties of organic liquids that are relevant to their use as
solvating solvents. Chemical Society Reviews, 22(6): 409-416.
22.
Alder,
C. M., Hayler, J. D., Henderson, R. K., Redman, A. M., Shukla, L., Shuster, L.
E. and Sneddon, H. F. (2016). Updating and further expanding GSK's solvent
sustainability guide. Green Chemistry, 18 (13): 3879-3890.
23.
Hellsten,
S., Qu, H. and Louhi‐Kultanen, M. (2011). Screening of binary solvent
mixtures and solvate formation of indomethacin. Chemical Engineering &
Technology, 34(10): 1667-1674.