Malaysian
Journal of Analytical Sciences Vol 26 No 1
(2022): 164 - 175
TEMPERATURE EFFECT ON THE
ENCAPSULATION OF THE DRUG TETRACAINE HYDROCHLORIDE IN DIFFERENT CYCLODEXTRINS
(Kesan Suhu Terhadap Pengkapsulan Dadah
Tetrakain Hidroklorida dalam Siklodekstrin yang Berbeza)
Houria Boudjoras, Teffaha
Fergoug, Mansour Azayez*, Youcef Bouhadda, Noureddine Meddah-araibi, Cherifa Zelmat
Laboratory of Physical Chemistry of Macromolecular and Biological
Interfaces,
Faculty of Exact Sciences,
University of Mustapha Stambouli, Mascara, Algeria
*Corresponding
author: m.azayez@univ-mascara.dz
Received: 25 September 2021; Accepted: 30 December 2021;
Published: 25 February 2022
Abstract
The
encapsulation of tetrakain hydrochloride (TC-HCl) in α-cyclodextrin
(α-CD), β -cyclodextrin (β-CD) and
hydroxypropyl-β-cyclodextrin (HPβ-CD) has been studied by UV-Visible
at different temsperatures. The appearance of isosbestic points as well as
hyperchromic and bathochromic shifts on the different UV-Visible spectra
confirm the complexes formation. From the complexation constants values the
stability of the 1:1 type complexes is in the order of α-CD <
HPβ-CD < β-CD and decreases with increasing temperature for each
complex. All complexation processes are spontaneous, with a favorable enthalpic
contribution and an unfavorable entropic term as deduced from Van't Hoff plot
analysis. The negative values obtained for ∆Cpo indicate that the apolar
part of TC-HCl is encapsulated in the cavities of the CDs.
Keywords: UV-Vis
spectrophotometry, cyclodextrins, tetracaine drug, temperature effect, Van't
Hoff analysis
Abstrak
Pengkapsulan
tetrakain hidroklorida (TC, HCl) dalam α-siklodekstrin (α-CD),
β-sikloododekstrin (β-CD) dan hidroksipropil-β-siklodekstrin
(HPβ-CD) telah dikaji mengunakan spektrofotometri UV-cahaya nampak pada
suhu yang berbeza. Kemunculan titik isosbestik serta pergeseran hipokromik dan
bathokromik pada spektrum UV-cahaya nampak yang berbeza mengesahkan pembentukan
kompleks. Daripada nilai pemalar kompleks, kestabilan kompleks jenis 1: 1
berada dalam urutan α-CD <HPβ-CD <β-CD dan menurun dengan
kenaikan suhu bagi setiap kompleks. Semua proses pengkompleksan adalah spontan,
dengan sumbangan entalpik yang menggalakkan dan istilah entropik yang tidak
menguntungkan seperti hasil analisis plot Van't Hoff. Nilai negatif yang
diperoleh untuk ∆Cpo menunjukkan bahawa bahagian tidak berkutub TC-HCl
dikemas dalam rongga CD.
Kata
kunci: spektrofotometri
UV-cahaya nampak, siklodekstrin, dadah tetrakain, kesan suhu, analisis Van't
Hoff
Graphical Abstract
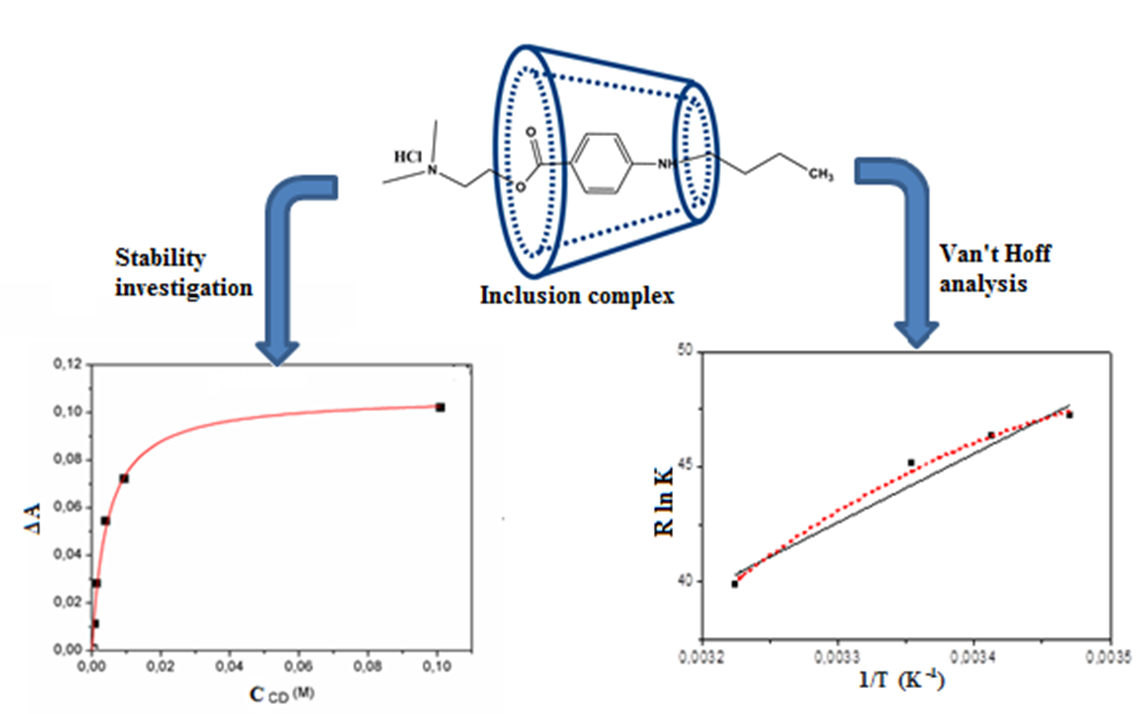
References
1. Mary Ann Vann,
M. D., Babatunde O. Ogunnaike, M. D. and Girish P. Joshi, M.B. (2007). Sedation and
anesthesia care for ophthalmologic surgery during local/regional anesthesia. Anesthesiology, 107(3):
502-508.
2.
, , B. and (2007).
Perioperative myocardial ischemia in cataract surgery patients: general versus
local anesthesia. Anesthesia & Analgesia, 91(6):
1415-1419.
3. Cherobin, A. C. F. P. and Tavares, G. T.
(2020). Safety of local anesthetics. Anais
Brasileiros de Dermatologia, 95 (1):82-90.
4. Ariga, K. and Kunitake, T. (2006). Supramolecular
chemistry-fundamentals and applications. Springer-Verlag
Berlin, Heidelberg: pp. 207-238.
5. (2006).
Multifunctional
nanocarriers. Advanced Drug Delivery Reviews, 58(14): 1532-1555.
6. Loftsson, T.
and Masson, M. (2001). Cyclodextrin in topical drug formulation: Theory and
practice. International Journal of Pharmaceutics, 225(1-2): 15-30.
7.
Fromming,
K. H. and Szejtli, J. (1994). Cyclodextrin
in pharmacy. Klumer Academies
Publishers, Dordrecht: pp. 1-18.
8. Martin Del
Valle, E. M. (2004). Cyclodextrins and their uses: A review. Process
Biochemistry, 39: 1033-1046.
9. Maheriya, P. M. (2017). Cyclodextrin: A promising candidate in
enhancing oral bioavailability of poorly water soluble drugs. Bioequivalence
&Bioavailability, 3(3):
60-63.
10.
Junquera,
E. and Aicart, E. (1997). Potentiometric study of the encapsulation of
ketoprophen by hydroxypropyl-β-cyclodextrin. Temperature, solvent, and
salt effects. Journal Physical Chemistry. B, 101(36), 7163-7171.
11. Astray, G.,
Mejuto, J. C.,
Morales, J., Rial-Otero, R. and Simal-Gandara, J. (2010). Factors controlling flavors binding
constants to cyclodextrins and their applications in foods. Food Research International, 43
(4): 1212-1218.
12. Rekharsky, M. V. and Inoue, Y. (1998).
Complexation thermodynamics of cyclodextrins. Chemical Reviews, 98(5): 1875-1918.
13. , , A. C. F. and M. A. (2019). Drug delivery systems: Study of
inclusion complex formation between methylxanthines and cyclodextrins and their
thermodynamic and transport properties. Biomolecules, 9(5): 196-216.
14.
Hugh, C., Hemmings, Jr. and Talmage D. E. (2019).
Pharmacology and physiology for anesthesia: Foundations and clinical
application, Elsevier, Philadelphia. pp: 20-43.
15.
Shibata,
A., Ikawa, K. and Terada, H. (1995). Site of action of
the local anesthetic tetracaine in a phosphatidylcholine bilayer with
incorporated cardiolipin. Biophysical Journal, 69(2): 470-477.
16. Schalley, C. (2007). Analytical methods in supramolecular
chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. pp: 419-471.
17. Nouiri, M. A., Fergoug, T., Azayez, M.,
Boujores, H., Zelmat, C. and Bouhadda, Y. (2017). Experimental and theoretical
study of tetracaine-hydrochloride β-cyclodextrin complexation. Journal
of Materials Environmental Sciences, 8(5): 1589-1598.
18. Merino,
C., Junquera, E., Jimenez-Barbero, J. and Aicart, E.
(2000). Effect of the presence of β-cyclodextrin on the solution behavior
of procaine hydrochloride. spectroscopic and thermodynamic studies. Langmuir,
16(4): 1557-1565.
19.
Mura, P. (2014). Analytical techniques for
characterization of cyclodextrin complexes in aqueous solution: A review. Journal
of Pharmaceutical and Biomedical Analysis, 101: 238-250.
20. Garcia, I., Brandariz, I.
and Iglesias, E. (2010). Fluorescence study of tetracaine-cyclodextrin
inclusion complexes. Journal of Supramolecular Chemistry, 22(4): 228-236.
21. Takisawa, N.,
Shirahama, K. and Tanaka, I. (1993). Interactions of amphiphilic drugs with α-, β-,
and γ- cyclodextrins. Colloid and Polymer Science,
271: 499-506.
22. Cano, J. Rodriguez, A. Aicart, E.
and Junquera, E. (2007). Temperature effect on the complex formation
between tricyclic antidepressant drugs (amitriptyline or imipramine) and
hydroxypropyl-β-cyclodextrin in water. Journal of Inclusion Phenomena
and Macrocyclic Chemistry, 59: 279-285.
23. Theoretical
studies on water-tetracaine interaction.Journal
Quantum Chemistry, 106: 1277-1282.
24.
Density
functional and molecular dynamics simulations of local anesthetics in 0.9% NaCl
solution. Molecular Simulation, 33(14): 1135-1141.