Malaysian
Journal of Analytical Sciences Vol 26 No 1
(2022): 152 - 163
CATALYTIC
NEUTRALIZATION OF ACIDIC PETROLEUM CRUDE OIL UTILIZING 2-METHYLIMIDAZOLE WITH
ADDITION OF Cu/Ce(10:90)/Al2O3 CATALYST
(Peneutralan
Pemangkin Minyak Mentah Petroleum Berasid Menggunakan 2-Metilimidazol dengan
Tambahan Mangkin Cu/Ce(10:90)/Al2O3)
Norshahidatul
Akmar Mohd Shohaimi1*, Noraini Safar Che Harun1, Hisyam
Saufi Tajudin1, Wan
Azelee Wan Abu Bakar2, Nurasmat Mohd Shukri3, Nor Hakimin
Abdullah4, Ahmad Zamani Ab Halim5
1Faculty of Applied Sciences,
Universiti Teknologi MARA Pahang,
Bandar Tun Abdul Razak Jengka, Pahang, Malaysia
2Faculty of Sciences,
Universiti Teknologi Malaysia, Johor
Bahru, Johor, Malaysia
3School of Health Sciences,
Universiti Sains Malaysia, Health
Campus, 16150 Kubang Kerian, Kelantan
4Advanced Materials Research Centre
(AMRC), Faculty of Bioengineering and Technology,
Universiti Malaysia Kelantan, Locked
Bag 100, 17600 Jeli, Kelantan
5Faculty of Industrial Sciences &
Technology,
Universiti Malaysia Pahang, 26300 Gambang,
Kuantan, Pahang, Malaysia
*Corresponding author: akmarshohaimi@uitm.edu.my
Received: 22 December 2021; Accepted: 3 February 2022;
Published: 25 February 2022
Abstract
The
presence of naphthenic acid (NA) in crude oil leads to corrosion problems
within oil refineries which may increase the maintenance cost and produce lower
quality of crude oil. The objective of this study is to reduce the total acid
number (TAN) of Petronas Penapisan Melaka (PPM)'s crude oil (TAN = 2.43 mgKOH/g)
using 2-methylimidazole with the aid of Cu/Ce (10:90)/Al2O3
catalyst through the catalytic neutralization technique. A 10% of 2-methylimidazole
in ethanol solution was used as the acid removal agent. Cerium oxide based
catalysts with copper as a dopant were supported onto alumina and calcined at
different calcination temperatures of 800 ℃, 900 ℃ and 1000
℃. The potential catalyst was characterized by using TGA-DTG, FTIR and
XRD for its physicochemical properties. The results showed TAN was reduced to
0.53 mg KOH/g with 78.2% reduction at catalyst calcination temperature of 900
℃, 0.5% catalyst loading, reaction temperature of 27 ℃ and 10
minutes reaction time. The small particle size of catalyst calcined at 900
℃ which was 18.02 nm led to bigger surface areas that enhanced the
neutralization process. These structural properties contributed to the
excellent catalytic performance which removed the NAs in the PPM's crude oil
and concurrently reduced the TAN value below than one.
Keywords: catalyst, catalytic neutralization, crude oil,
naphthenic acid
Abstrak
Kehadiran asid naftenik (NA)
dalam minyak mentah membawa kepada masalah kakisan dalam kilang penapisan
minyak yang boleh meningkatkan kos penyelenggaraan dan menghasilkan minyak
mentah yang berkualiti rendah. Objektif kajian ini adalah untuk mengurangkan
jumlah asid (TAN) minyak mentah Petronas Penapisan Melaka (PPM) (TAN = 2.43 mgKOH/g)
menggunakan 2-metilimidazol dengan bantuan mangkin Cu/Ce
(10:90)/ Al2O3 melalui teknik peneutralan pemangkin. 10%
daripada 2-metilimidazol dalam larutan etanol digunakan sebagai agen penyingkiran
asid. Pemangkin berasaskan serium oksida dengan kuprum sebagai dopan disokong
pada alumina dan dikalsinkan pada suhu pengkalsinan berbeza 800 ℃, 900
℃ dan 1000 ℃. Mangkin berpotensi dicirikan dengan menggunakan
TGA-DTG, FTIR dan XRD untuk sifat fizikokimianya. Keputusan menunjukkan TAN dikurangkan
kepada 0.53 mg KOH/g dengan pengurangan 78.2% pada suhu pengkalsinan mangkin
900 ℃, 0.5% pemuatan mangkin, suhu tindak balas 27 ℃ dan 10 minit
masa tindak balas. Saiz zarah kecil pemangkin yang dikalsinkan pada 900 ℃
iaitu 18.02 nm membawa kepada kawasan permukaan yang lebih besar yang
meningkatkan proses peneutralan. Ciri-ciri struktur ini menyumbang kepada
prestasi pemangkin yang sangat baik yang mengeluarkan NA dalam minyak mentah
PPM dan secara serentak mengurangkan nilai TAN di bawah satu.
Kata kunci: mangkin,
peneutralan pemangkin, minyak mentah, asid naftenik
Graphical Abstract
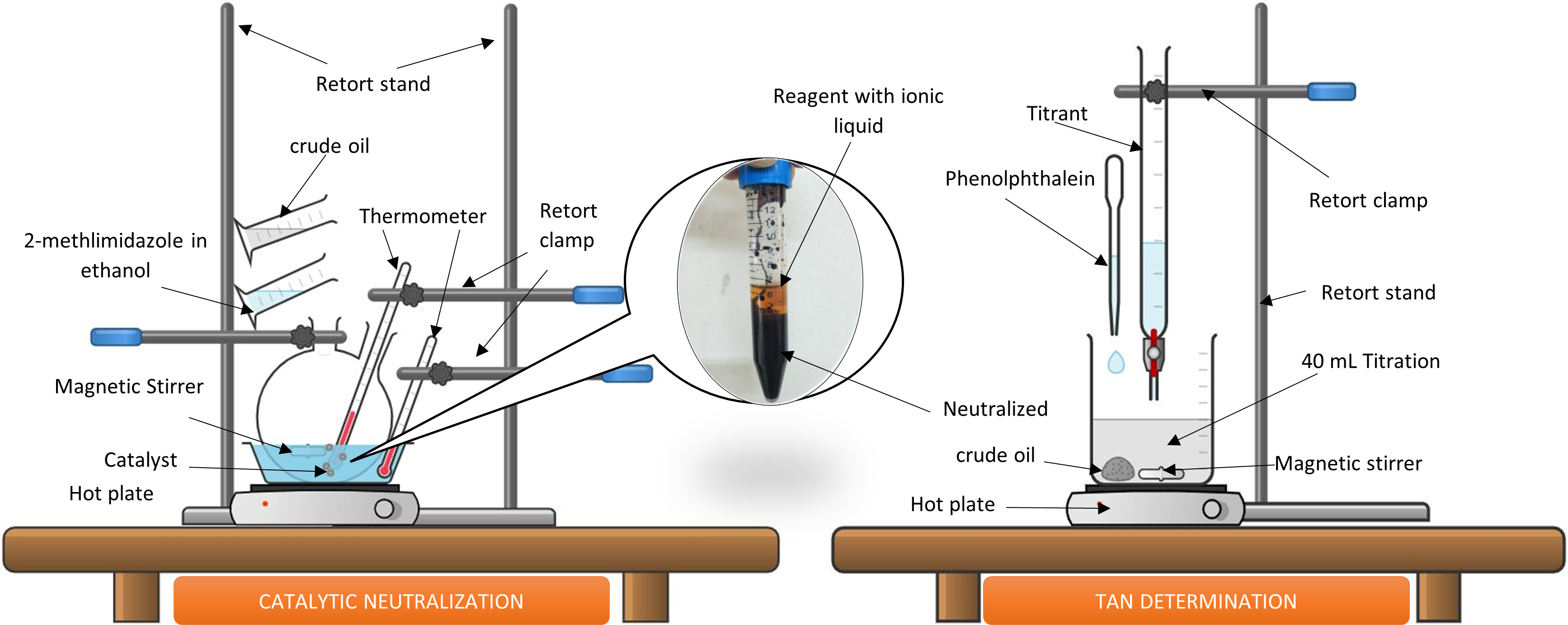
References
1.
Shohaimi, N. A., Mohd Halim, N. S., Ab Halim, A. Z., Mohd
Shukri, N. and Abdullah, N. H. (2020). Catalytic study of Ni/Ce/Al2O3
and Ni/Ca/Al2O3 on the removal of naphthenic acid from
petroleum crude oil utilizing sodium thiocyanate in ethanol. Petroleum
Science and Technology, 38(6): 602-608.
2.
Sun, Y. and Shi, L. (2012). Basic ionic liquids with
imidazole anion: New reagents to remove naphthenic acids from crude oil with
high total acid number. Fuel, 99: 83-87.
3.
Cho, K., Rana, B. S., Cho, D. W., Beum, H. T., Kim, C. H.
and Kim, J. N. (2020). Catalytic removal of naphthenic acids over Co-Mo/γ-Al2O3
catalyst to reduce total acid number (TAN) of highly acidic crude oil. Applied
Catalysis A: General, 606: 117835.
4.
Shukri, N. M., Bakar, W. A., Jaafar, J. and Majid, Z.
A. (2015). Removal of naphthenic acids
from high acidity Korean crude oil utilizing catalytic deacidification method. Journal
of Industrial and Engineering Chemistry. 28: 110-116.
5.
Wu, C., De Visscher, A. and Gates, I. D. (2019). On
naphthenic acids removal from crude oil and oil sands process-affected water. Fuel,
253: 1229-1246.
6.
Shukri, N. M., Bakar, W. A., Jaafar, J. and Majid, Z.
A. (2015b) Optimization of basic
catalyst with ammoniated polyethylene glycol for the removal of naphthenic acid
from petroleum crude oil by Box-Behnken design. Clean Techn Environ Policy
Clean Technologies and Environmental Policy, 17(8), 2387-2400.
7.
Zamani, A. H., Shohaimi, N. A. M., Rosid, S. J. M.,
Abdullah, N. H. and Shukri, N. M. (2019). Enhanced low temperature reaction for
the CO2 methanation over Ru promoted Cu/Mn on alumina support
catalyst using double reactor system. Journal of the Taiwan Institute of
Chemical Engineers, 96: 400-408.
8.
Shohaimi, N. A. M., and Marodzi, N. F. S. (2018).
Transesterification of waste cooking oil in biodiesel production utilizing
CaO/Al2O3 Heterogeneous Catalyst. Malaysian Journal of
Analytical Sciences, 22(1): 157-165.
9.
Aziz, N. H., Shohaimi, N. A. M. and Che Harun, N. S.
(2021). The effectiveness of Ni/Ce/Al2O3 catalyst in the
extraction of naphthenic acids from acidic crude oil. Materials Science
Forum, 1025: pp. 284-289.
10.
Shohaimi, N. A. M, Jelani, N., Ab Halim, A. Z., Abdullah,
N. H. and Shukri, N. M. (2021). Catalytic neutralization of naphthenic acid
from petroleum crude oil by using cerium oxide catalyst and 2-methylimidazole
in polyethylene glycol. Recent Innovations in Chemical Engineering,
14(3): 219-227.
11.
Shohaimi, N. A. M., Jaafar, J. and Bakar, W. A. W. A.
(2015). Catalytic deacidification optimization of Korean crude oil based on
response surface methodology. Clean Technologies and Environmental Policy,
17(6), 1513-1522.
12.
Shohaimi N.A.M, Bakar, W. A. W. A. and Jaafar, J. (2014). Catalytic
neutralization of acidic crude oil utilizing ammonia in ethylene glycol basic
solution. Journal of Industrial and Engineering Chemistry, 20(4):
2086-2094 .
13.
Shi, L. J., Shen, B. X., Wang, G. Q. (2008). Removal of
naphthenic acids from beijiang crude oil by forming ionic liquids. Energy
& Fuels Energy Fuels. 22(6): 4177-4181.
14.
Shohaimi, N. A. M., Bakar, W. A. W. A. and Jaafar, J.
(2014). Catalytic neutralization method for naphthenic acid removal in crude
oil by alumina supported Ca and Ba catalysts. Petroleum Science and
Technology, 32(19): 2365-2375.
15.
Dias, A. P. S., Bernardo, J., Felizardo, P. and Neiva
Correi, M. J. (2012). Biodiesel production over thermal activated cerium
modified Mg-Al hydrotalcites. Energy. 41: 344-353.
16.
Alouche, A. (2008). Preparation and characterization of
copper and/or cerium catalysts supported on alumina or ceria. Jordan Journal
of Mechanical and Industrial Engineering, 2: 111-116.
17.
Bakar, W. A. W. A., Ali, R., Sulaiman, N. and Rahim, H. A.
(2010). Manganese oxide doped noble metals supported catalyst for carbon
dioxide methanation reaction. Iranian Journal of Science and Technology
Transactions. 17: 115-123.
18.
Lamonier, C., Bennani, A., D'Huysser, A., Aboukais, A. and
Wrobel, G. (1996). Evidence for different copper species in precursors of
copper-cerium oxide catalysts for hydrogenation reactions: An X-ray
diffraction, EPR and X-ray photoelectron spectroscopy study. Journal of
Chemical Society Faraday Transaction, 92(l): 131-136.
19.
Bueno-Ferrer, C., Parres-Esclapez, S., Lozano-Castello, D.
and Bueno-Lopez, A., (2010). Relationship between surface area and crystal size
of pure and doped cerium oxides. Journal of Rare Earths, 28: 647-656.
20.
Roy, A. and Bhattacharya, J., (2011). Microwave-assisted
synthesis and characterization of CaO nanoparticles. International Journal
Nanoscience, 10(41): 3-8.
21.
Li, X., Zhu, J., Liu, Q. and Wu, B., (2013). The removal
of naphthenic acids from dewaxed VGO via esterification catalyzed by Mg�Al
hydrotalcite. Fuel Processing Technology, 111: 68-77.