Malaysian
Journal of Analytical Sciences Vol 26 No 3
(2022): 664 - 683
ENHANCING THE OXYGEN REDUCTION REACTION OF LOW-PLATINUM AND
NON-PLATINUM CATALYSTS FOR FUEL CELL APPLICATIONS
(Peningkatan Tindak Balas Penurunan Oksigen Mangkin
Platinum Bermuatan Rendah dan Bebas Platinum Untuk Aplikasi Sel Bahan Api)
Kazi Rumanna Rahman1, Kuan Ying Kok2,
Nor Azillah Fatimah Othman3, Wai Yin Wong1, Kean Long Lim1*
1Fuel
Cell Institute,
Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor,
Malaysia
2 Industrial
Technology Division
3Radiation
Processing Technology Division
Malaysia Nuclear Agency, 43000, Kajang, Selangor, Malaysia
*Corresponding
author email: kllim@ukm.edu.my
Received: 13 December 2021; Accepted: 6 March 2022;
Published: 27 June 2022
Abstract
Nowadays, research efforts are focused on developing low-Pt and non-Pt
catalysts for ORR. The addition of Pt-group metal (PGM) in pure Pt to form
Pt-PGM catalysts exhibits better ORR performance than pure PGM catalysts and
Pt/C. More than 20 wt.% of Pt loading is required in Pt-PGM alloys for
improving catalytic activity. Studies have also shown that the addition of
transition metal (TM), which has a smaller atomic size, into PGM reduced the
bond distance between two metallic atoms and geometric parameters, thus
remarkably improving the catalytic stability and ORR activity. However, TM
based catalysts should be supported on nitrogen-doped carbon with high surface
area to attain high ORR activity. A large surface area and high electronic
conductivity of carbon support also facilitate the ORR activity. Performances
of alloy catalysts are directly related to their synthesis temperature and
structural properties. Designing the core-shell combinations and controlling
the shell thickness is one of the structural strategies in enhancing mass
activity and durability. Lately, irradiation techniques are used to modify the
physicochemical properties. Nevertheless, TM-based catalysts are usually stable
in alkaline solutions but not in acidic solutions. This review focuses on the
strategies to develop cost-effective catalysts from low-platinum and
non-platinum catalysts with enhanced ORR activity.
Keywords: oxygen reduction reaction,
platinum group metal alloys, transition metal catalysts, synthesis techniques
Abstrak
Pada masa kini, usaha penyelidikan tertumpu kepada
pembangunan mangkin platinum (Pt) bermuatan rendah dan bebas platinum untuk
tindak balas penurunan oksigen (ORR). Penambahan logam kumpulan Pt (PGM) dalam
Pt tulen untuk membentuk Pt-PGM menunjukkan prestasi ORR yang lebih baik
daripada prestasi mangkin PGM tulen dan Pt/C. Sebanyak lebih daripada 20 wt.%
muatan Pt adalah diperlukan dalam aloi Pt-PGM untuk meningkatkan aktiviti
pemangkinan. Kajian telah menunjukkan penambahan logam peralihan (TM) yang mempunyai
saiz atom yang lebih kecil ke dalam PGM mengurangkan jarak antara dua atom
logam dan parameter geometri sekali gus meningkatkan kestabilan pemangkinan dan
aktiviti ORR. Namun demikian, mangkin berasakan TM perlu disokong dengan karbon
terdop nitrogen yang berpermukaan yang luas untuk mencapai aktiviti ORR yang
tinggi. Penyokong karbon yang berpemukaan luas dan mempunyai kekonduksian
elektronik yang tinggi juga memudahkan
aktiviti ORR. Prestasi mangkin aloi adalah berkait langsung dengan suhu
sintensi dan sifat strukturnya. Mereka bentuk gabungan teras-cangkerang dan
mengawal ketebalan cangkerang adalah salah satu strategik penstrukturan dalam
meningkatkan aktiviti jisim dan ketahanan. Akhir-akhir ini, teknik-teknik
penyinaran digunakan ubah mengubahsuai sifat fizikokimia. Namun demikian,
mangkin berasaskan TM biasanya stabil dalam larutan alkali tetapi tidak dalam
larutan asid. Ulasan ini berfokus pada strategik untuk membangunkan magkin
berkos efektif daripada mangkin Pt bermuatan rendah dan bebas platinum dengan
aktitiviti ORR yang dipertingkatkan.
Kata
kunci:
tindak balas penurunan oksigen, logam aloi kumpulan platinum, mangkin
logam peralihan, kaedah sintesis
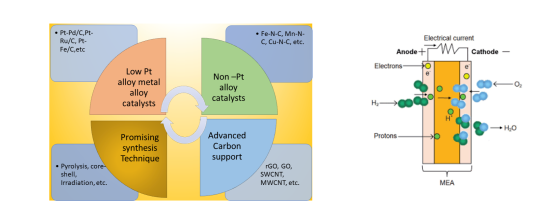
Graphical Abstract
References
1. Rajapakse,
R. M. G., Senarathna, K. G. C., Kondo, A., Jayawardena, P. S. and Shimomura, M.
(2015). Extremely low-cost alternative for the oxygen reduction catalyst of
fuel cell. Advances in Automobile Engineering, 4(1): 1.
2. Zhang,
Y., Huang, N., Zhou, F., He, Q. and Zhan, S. (2018). Research on the oxygen
reduction reaction (ORR) mechanism of g-C3N4 doped by Ag
based on first-principles calculations. Journal of the Chinese Chemical
Society, 65(12): 1431-1436.
3. She,
Y., Chen, J., Zhang, C., Lu, Z., Ni, M., Sit, P. H. L. and Leung, M. K. H.
(2017). Oxygen Reduction reaction mechanism of nitrogen-doped graphene derived
from ionic liquid. Energy Procedia, 142: 1319-1326.
4. Hazarika,
K. K., Goswami, C., Saikia, H., Borah, B. J. and Bharali, P. (2018). Cubic Mn2O3
nanoparticles on carbon as bifunctional electrocatalyst for oxygen reduction
and oxygen evolution reactions. Molecular Catalysis, 451: 153-160.
5. Laurent-Brocq,
M., Job, N., Eskenazi, D. and Pireaux, J. J. (2014). Pt/C catalyst for PEM fuel
cells: Control of Pt nanoparticles characteristics through a novel plasma
deposition method. Applied Catalysis B: Environmental, 147: 453-463.
6. Shao-Horn,
Y., Sheng, W. C., Chen, S., Ferreira, P. J., Holby, E. F. and Morgan, D. (2007).
Instability of supported platinum nanoparticles in low-temperature fuel cells. Topics
in Catalysis, 46(3–4): 285–305.
7. Dombrovskis,
J. K. and Palmqvist, A. E. C. (2016). Recent progress in synthesis,
characterization and evaluation of non-precious metal catalysts for the oxygen
reduction reaction. Fuel Cells, 16(1): 4-22.
8. Holton,
O. and Stevenson, J. (2013). The role of platinum in proton exchange membrane
fuel cells - Johnson Matthey technology review. Platinum Metals Reviews,
57(4): 259-271.
9. Mustain,
W. E., Shrestha, S., Ashegi, S., Timbro, J., Lang, C. M. and Mustain, W. E.
(2011). ORR and fuel cell performance of Pt supported on N-functionalized
mesoporous carbon, ECS Transactions, 41(1): 1183.
10. Ghosh,
S., Mondal, S. and Retna Raj, C. (2014). Carbon nanotube-supported dendritic
Pt-on-Pd nanostructures: Growth mechanism and electrocatalytic activity towards
oxygen reduction reaction. Journal of Materials Chemistry A, 2(7):
2233-2239.
11. Guha,
A., Lu, W., Zawodzinski, T. A. and Schiraldi, D. A. (2007). Surface-modified
carbons as platinum catalyst support for PEM fuel cells, Carbon, 45:
1506-1517.
12. Mun,
Y., Lee, S., Kim, K., Kim, S., Lee, S., Han, J. W. and Lee, J. (2019).
Versatile strategy for tuning ORR activity of a single Fe-N4 site by
controlling electron-withdrawing/donating properties of a carbon plane. Journal
of the American Chemical Society, 141(15): 6254-6262.
13. Kodali,
M., Santoro, C., Serov, A., Kabir, S., Artyushkova, K., Matanovic, I. and
Atanassov, P. (2017). Air breathing cathodes for microbial fuel cell using Mn-,
Fe-, Co- and Ni-containing platinum group metal-free catalysts. Electrochimica
Acta, 231: 115-124.
14. Song,
M., Song, Y., Sha, W., Xu, B., Guo, J. and Wu, Y. (2020). Recent advances in
non-precious transition metal/nitrogen-doped carbon for oxygen reduction
electrocatalyst. Catalysts, 10(1): 141.
15. Mao,
J., Liu, P., Du, C., Liang, D., Yan, J. and Song, W. (2019). Tailoring 2D MoS2
heterointerfaces for promising oxygen reduction reaction
electrocatalysis. Journal of Materials Chemistry A, 7(15): 8785-8789.
16. Ma,
R., Lin, G., Zhou, Y., Liu, Q., Zhang, T., Shan, G. and Wang, J. (2019). A
review of oxygen reduction mechanisms for metal-free carbon-based
electrocatalysts. NPJ Computational Materials, 5(1): 1-15.
17. Tang,
Z., Wu, W. and Wang, K. (2018). Oxygen reduction reaction catalyzed by noble
metal clusters. Catalysts, 8(2): 65.
18. Kim,
J. Y., Oh, T. K., Shin, Y., Bonnett, J. and Weil, K. S. (2011). A novel
non-platinum group electrocatalyst for PEM fuel cell application. International
Journal of Hydrogen Energy, 36(7): 4557-4564.
19. Wang,
B. (2005). Recent development of non-platinum catalysts for oxygen reduction
reaction. Journal of Power Sources, 152(1–2): 1-15.
20. Sui,
S., Wang, X., Zhou, X., Su, Y., Riffat, S. and Liu, C. J. (2017). A
comprehensive review of Pt electrocatalysts for the oxygen reduction reaction:
Nanostructure, activity, mechanism and carbon support in PEM fuel cells. Journal
of Materials Chemistry A, 5(5): 1808-1825.
21. Raciti,
D., Kubal, J., Ma, C., Barclay, M., Gonzalez, M., Chi, M. and Wang, C. (2016).
Pt3Re alloy nanoparticles as electrocatalysts for the oxygen reduction
reaction. Nano Energy, 20: 202-211.
22. Hyun,
K., Lee, J. H., Yoon, C. W. and Kwon, Y. (2013). The effect of platinum based
bimetallic electrocatalysts on oxygen reduction reaction of proton exchange
membrane fuel cells. International Journal of Electrochemical Science,
8(10): 11752-11767.
23. Tian,
J., Wu, W., Tang, Z., Wu, Y., Burns, R., Tichnell, B. and Chen, S. (2018).
Oxygen reduction reaction and hydrogen evolution reaction catalyzed by Pd–Ru
nanoparticles encapsulated in porous carbon nanosheets. Catalysts, 8(8):
1-15.
24. Zhou,
Z. M., Shao, Z. G., Qin, X. P., Chen, X. G., Wei, Z. D. and Yi, B. L. (2010).
Durability study of Pt-Pd/C as PEMFC cathode catalyst. International Journal
of Hydrogen Energy, 35(4): 1719-1726.
25. Wang,
W., Wang, Z., Wang, J., Zhong, C. J. and Liu, C. J. (2017). Highly active and
stable Pt–Pd Alloy catalysts synthesized by room-temperature electron reduction
for oxygen reduction reaction. Advanced Science, 4(4): 1-9.
26. Yusof,
M. S. M., Jalil, A. A., Ahmad, A., Triwahyono, S., Othman, M. H. D., Abdullah,
T. A. T. and Nabgan, W. (2019). Effect of Pt–Pd/C coupled catalyst loading and
polybenzimidazole ionomer binder on oxygen reduction reaction in
high-temperature PEMFC. International Journal of Hydrogen Energy, 2019:
20760-20769.
27. Thanasilp,
S. and Hunsom, M. (2011). Effect of Pt: Pd atomic ratio in Pt-Pd/C
electrocatalyst-coated membrane on the electrocatalytic activity of ORR in PEM
fuel cells. Renewable Energy, 36(6): 1795-1801.
28. Jackson,
C., Conrad, O. and Levecque, P. (2017). Systematic study of Pt-Ru/C catalysts
prepared by chemical deposition for direct methanol fuel cells. Electrocatalysis,
8(3): 224-234.
29. Huang,
H., Zhu, J., Li, D., Shen, C., Li, M., Zhang, X. and Wu, Y. (2017). Pt
nanoparticles grown on 3D RuO2-modified graphene architectures for
highly efficient methanol oxidation. Journal of Materials Chemistry A,
5(9): 4560-4567.
30. Yang,
G., Sun, Y., Lv, P., Zhen, F., Cao, X., Chen, X. and Kong, X. (2016).
Preparation of Pt–Ru/C as an oxygen-reduction electrocatalyst in microbial fuel
cells for wastewater treatment. Catalysts, 6(10): 150.
31. Jackson,
A., Strickler, A., Higgins, D. and Jaramillo, T. F. (2018). Engineering Ru@Pt
core-shell catalysts for enhanced electrochemical oxygen reduction mass activity
and stability. Nanomaterials, 8(1): 38.
32. Tolmachev,
Y. V. and Petrii, O. A. (2017). Pt–Ru electrocatalysts for fuel cells:
developments in the last decade. Journal of Solid State Electrochemistry,
21(3): 613-639.
33. Durst,
J., Simon, C., Hasché, F. and Gasteiger, H. A. (2015). Hydrogen oxidation and
evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd
electrocatalysts in acidic media. Journal of The Electrochemical Society,
162(1): F190-F203.
34. Zeng,
M., Wang, X. X., Tan, Z. H., Huang, X. X. and Wang, J. N. (2014). Remarkable
durability of Pt-Ir alloy catalysts supported on graphitic carbon nanocages. Journal
of Power Sources, 264: 272-281.
35. Zheng,
H. B., An, L., Zheng, Y., Qu, C., Fang, Y., Liu, Q. and Dang, D. (2018). Tuning
the catalytic activity of Ir@Pt nanoparticles through controlling ir core size
on cathode performance for PEM fuel cell application. Frontiers in Chemistry,
6(7): 1-7.
36. Fang,
D., Tang, X., Yang, L., Xu, D., Zhang, H., Sun, S. and Yi, B. (2019). Facile
synthesis of Pt-decorated Ir black as a bifunctional oxygen catalyst for oxygen
reduction and evolution reactions. Nanoscale, 11(18): 9091-9102.
37. Zhu,
J., Elnabawy, A. O., Lyu, Z., Xie, M., Murray, E. A., Chen, Z., Xia, Y. (2019).
Facet-controlled Pt–Ir nanocrystals with substantially enhanced activity and
durability towards oxygen reduction. Materials Today, 2019: 1-9.
38. Lin,
C., Wu, G., Li, H., Geng, Y., Xie, G., Yang, J. and Jin, J. (2017). Rh
nanoparticles supported on ultrathin carbon nanosheets for high-performance
oxygen reduction reaction and catalytic hydrogenation. Nanoscale, 9(5):
1834-1839.
39. Paál,
Z., Gyorffy, N., Wootsch, A., Tóth, L., Bakos, I., Szabó, S. and Schlögl, R.
(2007). Preparation, physical characterization and catalytic properties of
unsupported Pt-Rh catalyst. Journal of Catalysis, 250(2):
254-263.
40. Narayanamoorthy,
B., Datta, K. K. R., Eswaramoorthy, M. and Balaji, S. (2014). Self-stabilized
Pt–Rh bimetallic nanoclusters as durable electrocatalysts for dioxygen
reduction in PEM fuel cells. RSC Advances, 4(98): 55571-55579.
41. Goswami,
C., Hazarika, K. K. and Bharali, P. (2018). Transition metal oxide
nanocatalysts for oxygen reduction reaction. Materials Science for Energy
Technologies, 1(2): 117–128.
42. Xin,
L., Zhang, Z., Wang, Z., Qi, J. and Li, W. (2013). Carbon supported Ag
nanoparticles as high performance cathode catalyst for H2/O2
anion exchange membrane fuel cell. Frontiers in Chemistry, 1: 16.
43. Esfandiari,
A., Kazemeini, M. and Bastani, D. (2016). Synthesis, characterization and
performance determination of an Ag@Pt/C electrocatalyst for the ORR in a PEM
fuel cell. International Journal of Hydrogen Energy, 41(45):
20720-20730.
44. Chiwata,
M., Yano, H., Ogawa, S., Watanabe, M., Iiyama, A. and Uchida, H. (2016). Oxygen
reduction reaction activity of carbon-supported Pt-Fe, Pt-Co, and Pt-Ni alloys
with stabilized Pt-skin layers. Electrochemistry, 84(3), 133–137.
45. Li,
W., Pan, Z., Huang, Z., Zhou, Q., Xu, Y., Wu, S., Hu, G. (2018). Pt
nanoparticles supported on titanium iron nitride nanotubes prepared as a
superior electrocatalysts for methanol electrooxidation. International
Journal of Hydrogen Energy, 43(20): 9777-9786.
46. Termpornvithit,
C., Chewasatn, N. and Hunsom, M. (2012). Stability of Pt-Co/C and Pt-Pd/C based
oxygen reduction reaction electrocatalysts prepared at a low temperature by a
combined impregnation and seeding process in PEM fuel cells. Journal of
Applied Electrochemistry, 42(3): 169-178.
47. Cui,
Y., Wu, Y., Wang, Z., Yao, X., Wei, Y., Kang, Y., Gan, L. (2020). Mitigating
metal dissolution and redeposition of Pt-Co catalysts in PEM fuel cells:
Impacts of structural ordering and particle size. Journal of The
Electrochemical Society, 167(6): 064520.
48. Rohendi,
D., Rachmat, A. and Syarif, N. (2018). Fabrication and characterization of
Pt-Co/C catalyst for fuel cell electrode. Journal of Physics: Conference
Series, 1095(1): 012007.
49. Singh,
R. N. (2012). Preparation of bimetallic Pd-Co nanoparticles on graphene support
for use as methanol tolerant oxygen reduction electrocatalysts. Engineering,
Technology & Applied Science Research, 2(6): 295-301.
50. Ramli,
Z. A. C. and Kamarudin, S. K. (2018). Platinum-based catalysts on various
carbon supports and conducting polymers for direct methanol fuel cell
applications: a review. Nanoscale Research Letters, 13: 1-25.
51. Mechler,
A. K., Sahraie, N. R., Armel, V., Zitolo, A., Sougrati, M. T., Schwämmlein, J.
N. and Jaouen, F. (2018). Stabilization of iron-based fuel cell catalysts by
non-catalytic platinum. Journal of The Electrochemical Society, 165(13):
F1084–F1091.
52. Mohanraju,
K. and Cindrella, L. (2014). Impact of alloying and lattice strain on ORR
activity of Pt and Pd based ternary alloys with Fe and Co for proton exchange
membrane fuel cell applications. RSC Advances, 4(23): 11939-11947.
53. Li,
X. P., Xiang, X. D., Yang, H. Y., Wang, X. J., Tan, C. L. and Li, W. S. (2013).
Hydrogen tungsten bronze-supported platinum as electrocatalyst for methanol
oxidation. Fuel Cells, 13(2): 314-318.
54. Seselj,
N., Engelbrekt, C. and Zhang, J. (2015). Graphene-supported platinum catalysts
for fuel cells. Science Bulletin, 60(9): 864-876.
55. Lv,
H., Li, D., Strmcnik, D., Paulikas, A. P., Markovic, N. M. and Stamenkovic, V.
R. (2016). Recent advances in the design of tailored nanomaterials for
efficient oxygen reduction reaction. Nano Energy, 29: 149-165.
56. Thippani,
T., Mandal, S., Wang, G., Ramani, V. K. and Kothandaraman, R. (2016). Probing
oxygen reduction and oxygen evolution reactions on bifunctional non-precious
metal catalysts for metal-air batteries. RSC Advances, 6(75):
71122-71133.
57. Osgood,
H., Devaguptapu, S. V., Xu, H., Cho, J. and Wu, G. (2016). Transition metal
(Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional
catalysts in alkaline media. Nano Today, 11(5): 601-625.
58. Akbari,
A., Amini, M., Tarassoli, A., Eftekhari-Sis, B., Ghasemian, N. and Jabbari, E.
(2018). Transition metal oxide nanoparticles as efficient catalysts in
oxidation reactions. Nano-Structures and Nano-Objects, 14: 19-48.
59. Ren,
G., Gao, L., Teng, C., Li, Y., Yang, H., Shui, J. and Dai, L. (2018). Ancient
Chemistry “pharaoh’s Snakes” for Efficient Fe-/N-Doped Carbon Electrocatalysts.
ACS Applied Materials and Interfaces, 10(13): 10778–10785.
60. Gu,
L., Jiang, L., Li, X., Jin, J., Wang, J. and Sun, G. (2016). A Fe-N-C catalyst
with highly dispersed iron in carbon for oxygen reduction reaction and its
application in direct methanol fuel cells. Cuihua Xuebao/Chinese Journal of
Catalysis, 37(4): 539-548.
61. Park,
M., Lee, J., Hembram, K., Lee, K.-R., Han, S., Yoon, C. and Kim, J. (2016).
Oxygen reduction electrocatalysts based on coupled iron nitride nanoparticles
with nitrogen-doped carbon. Catalysts, 6(6): 86.
62. Rahman,
K. R., Kok, K. Y., Wong, W. Y., Yang, H. and Lim, K. L. (2021). Effect of iron
loading on the catalytic activity of Fe/N-doped reduced graphene oxide
catalysts via irradiation. Applied Sciences (Switzerland), 11(1): 1-10.
63. Xi,
J., Wang, F., Mei, R., Gong, Z., Fan, X., Yang, H. and Luo, Z. (2016).
Catalytic performance of a pyrolyzed graphene supported Fe-N-C composite and
its application for acid direct methanol fuel cells. RSC Advances,
6(93): 90797-90805.
64. Meng,
H., Chen, X., Gong, T., Liu, H., Liu, Y., Li, H. and Zhang, Y. (2019). N, P,
S/Fe-codoped carbon derived from feculae bombycis as an efficient
electrocatalyst for oxygen reduction reaction. ChemCatChem, 11(24):
6015-6021.
65. Basri,
S. and Kamarudin, S. K. (2018). Nanocatalyst FeN4/C molecular
orbital behaviour for oxygen reduction reaction (ORR) in cathode direct methano
fuel cell (DMFC). Jurnal Kejuruteraan, 1(2): 59-64.
66. Jiang,
W. J., Gu, L., Li, L., Zhang, Y., Zhang, X., Zhang, L. J. and Wan, L. J.
(2016). Understanding the high activity of Fe-N-C electrocatalysts in oxygen
reduction: Fe/Fe3C nanoparticles boost the activity of Fe-Nx. Journal
of the American Chemical Society, 138(10): 3570-3578.
67. Hossen,
M. M., Artyushkova, K., Atanassov, P. and Serov, A. (2018). Synthesis and characterization
of high performing Fe-N-C catalyst for oxygen reduction reaction (ORR) in
alkaline exchange membrane fuel cells. Journal of Power Sources, 375:
214-221.
68. Zhan,
Y., Xie, F., Zhang, H., Lin, Z., Huang, J., Zhang, W. and Meng, H. (2018). Non
noble metal catalyst for oxygen reduction reaction and its characterization by
simulated fuel cell test. Journal of The Electrochemical Society,
165(15): J3008-J3015.
69. Kim,
S., Park, H. and Li, O. L. (2020). Cobalt nanoparticles on plasma-controlled
nitrogen-doped carbon as high-performance ORR electrocatalyst for primary
Zn-Air battery. Nanomaterials, 10(2): 223.
70. Zang,
J., Wang, F., Cheng, Q., Wang, G., Ma, L., Chen, C. and Yang, H. (2020).
Cobalt/zinc dual-sites coordinated with nitrogen in nanofibers enabling efficient
and durable oxygen reduction reaction in acidic fuel cells. Journal of
Materials Chemistry A, 8(7): 3686-3691.
71. Grinberg,
V. A., Mayorova, N. A., Pasynskii, A. A., Modestov, A. D., Shiryaev, A. A.,
Vysotskii, V. V. and Nogai, A. S. (2018). Nanostructured platinum-free
catalysts of oxygen reduction based on metal chalcogenide cobalt clusters. Russian
Journal of Coordination Chemistry, 44(10): 589-595.
72. Winey,
K. I., Li, J., Doan-Nguyen, V. V. T., Murray, C. B., Su, D., Trigg, E. B. and
Agarwal, R. (2015). Synthesis and X-ray
characterization of cobalt phosphide (Co2P) nanorods for the oxygen
reduction reaction. ACS Nano, 9(8): 8108-8115.
73. Zhao,
H., Xing, T., Li, L., Geng, X., Guo, K., Sun, C. and An, B. (2019). Synthesis
of cobalt and nitrogen co-doped carbon nanotubes and its ORR activity as the
catalyst used in hydrogen fuel cells. International Journal of Hydrogen Energy,
44(46): 25180-25187.
74. Liang,
G., Huang, J., Li, J., Wu, Y., Huang, G., Jin, Y. Q., ... and Meng, H. (2020).
Improving the catalytic performance of Co/N/C catalyst for oxygen reduction
reaction by alloying with Fe. Journal of The Electrochemical Society,
167(10), 104502.
75. An,
L., Jiang, N., Li, B., Hua, S., Fu, Y., Liu, J. and Sun, Z. (2018). A highly
active and durable iron/cobalt alloy catalyst encapsulated in N-doped graphitic
carbon nanotubes for oxygen reduction reaction by a nanofibrous dicyandiamide
template. Journal of Materials Chemistry A, 6(14): 5962-5970.
76. Liu,
P., Ran, J., Xia, B., Xi, S., Gao, D. and Wang, J. (2020). Bifunctional oxygen
electrocatalyst of mesoporous Ni/NiO nanosheets for flexible rechargeable
Zn–Air batteries. Nano-Micro Letters, 12(1), 1–12.
77. Hao,
Y., Xu, Y., Liu, J. and Sun, X. (2017). Nickel-cobalt oxides supported on Co/N
decorated graphene as an excellent bifunctional oxygen catalyst. Journal of
Materials Chemistry A, 5(11): 5594-5600.
78. Trzes̈niewski,
B. J., Diaz-Morales, O., Vermaas, D. A., Longo, A., Bras, W., Koper, M. T. M.
and Smith, W. A. (2015). In situ observation of active oxygen species in
Fe-containing Ni-based oxygen evolution catalysts: The effect of pH on
electrochemical activity. Journal of the American Chemical Society,
137(48): 15112-15121.
79. Zhuang,
Z., Giles, S. A., Zheng, J., Jenness, G. R., Caratzoulas, S., Vlachos, D. G.
and Yan, Y. (2016). Nickel supported on nitrogen-doped carbon nanotubes as
hydrogen oxidation reaction catalyst in alkaline electrolyte. Nature
Communications, 7: 1–8.
80. Faubert,
P., Kondov, I., Qazzazie, D., Yurchenko, O. and Müller, C. (2018). A non-noble
Cr-Ni-based catalyst for the oxygen reduction reaction in alkaline polymer
electrolyte fuel cells. MRS Communications, 8(1): 160-167.
81. Kabir,
S., Lemire, K., Artyushkova, K., Roy, A., Odgaard, M., Schlueter, D. and Serov,
A. (2017). Platinum group metal-free NiMo hydrogen oxidation catalysts: High
performance and durability in alkaline exchange membrane fuel cells. Journal
of Materials Chemistry A, 5(46): 24433-24443.
82. Xie,
X., Liu, J., Li, T., Song, Y. and Wang, F. (2018). Post-formation
copper-nitrogen species on carbon black: their chemical structures and active
sites for oxygen reduction reaction. Chemistry - A European Journal, 24(39):
9968-9975.
83. Hamedi,
M., Wigenius, J., Tai, F. I., Björk, P. and Aili, D. (2010). Polypeptide-guided
assembly of conducting polymer nanocomposites. Nanoscale, 2(10):
2058-2061.
84. Kang,
Y. S., Heo, Y., Kim, P. and Yoo, S. J. (2017). Preparation and characterization
of Cu–N–C electrocatalysts for oxygen reduction reaction in alkaline anion
exchange membrane fuel cells. Journal of Industrial and Engineering
Chemistry, 52(3): 35-41.
85. He,
Q., Yang, X., Ren, X., Koel, B. E., Ramaswamy, N., Mukerjee, S. and Kostecki,
R. (2011). A novel CuFe-based catalyst for the oxygen reduction reaction in
alkaline media. Journal of Power Sources, 196(18): 7404-7410.
86. 86. Qiao, Y., Ni, Y., Kong, F., Li, R., Zhang, C.,
Kong, A. and Shan, Y. (2019). Pyrolytic carbon-coated Cu-Fe alloy nanoparticles
with high catalytic performance for oxygen electroreduction. Chemistry - An
Asian Journal, 14(15): 2676-2684.