Malaysian
Journal of Analytical Sciences Vol 26 No 2
(2022): 360 - 369
DEVELOPMENT AND OPTIMIZATION OF A RAPID
RESOLUTION LIQUID CHROMATOGRAPHY METHOD FOR CYANIDIN-3-O-GLUCOSIDE IN RAT PLASMA
(Pembangunan dan
Pengoptimuman Kaedah Kromatografi Cecair Resolusi Pantas untuk Sianidin-3-O-Glukosida
Klorida di dalam Plasma Tikus)
Nadiratul Asyikin Sauji1, Wan Amir
Nizam Wan Ahmad1, Liza Nordin2, Ruzilawati Abu Bakar3*
1Biomedicine Program, School of Health Sciences
2Department of Physiology, School of Medical
Sciences
3Department of Pharmacology, School of Medical
Sciences
Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan,
Malaysia
*Corresponding
authors: ruzila@usm.my
Received: 16 January 2022; Accepted: 15 March 2022;
Published: 28 April 2022
Abstract
The growing interest in anthocyanins in plants
has brought about the importance of investigating their pharmacological
properties. Sensitive and specific analytical methods are required to
accurately analyze the anthocyanins present in samples. One of the anthocyanins
found in plants is cyanidin-3-O-glucoside. The objective of this study was to
develop and optimize a rapid resolution liquid chromatography (RRLC) method for
cyanidin-3-O-glucoside determination in rat plasma. Spectrophotometric analysis
was performed to determine the best ultraviolet (UV) absorbance wavelength.
Liquid-liquid extraction (LLE) and solid-phase extraction (SPE) methods were
compared to determine the best extraction method for cyanidin-3-O-glucoside in
rat plasma samples. The effects of varying the type and proportion of organic
solvents, the type and concentration of buffer solutions, flow rates, column
temperatures, and UV wavelengths were examined. The optimized chromatographic
method for RRLC analysis of cyanidin-3-O-glucoside was a mobile phase
composition of 0.1% trifluoroacetic acid aqueous solution and acetonitrile in a
ratio of 81:19, respectively, with a 0.5 mL/min flow rate, at 30°C column
temperature and 525 nm detection wavelength. SPE was our choice of final
extraction method. Our findings revealed that the optimized RRLC method can be
used to determine cyanidin-3-O-glucoside in rat plasma.
Keywords: rapid
resolution liquid chromatography, method development, cyanidin-3-o-glucoside,
rat plasma
Abstrak
Kajian
antosianin dalam rosel yang semakin meluas mengetengahkan kepentingan analisis
sebatian tersebut untuk mengkaji ciri-ciri farmakologinya. Kaedah analisis yang
sensitif dan spesifik diperlukan untuk menganalisis antosianin yang terdapat
dalam sampel dengan tepat. Salah satu antosianin yang terdapat dalam tumbuhan
ialah sianidin-3-O-glukosida. Objektif kajian ini adalah untuk membangunkan dan
mengoptimumkan kaedah kromatografi cecair resolusi pantas (RRLC) untuk
sianidin-3-O-glukosida di dalam plasma tikus. Analisis spektrofotometri
dilakukan untuk memilih penyerapan ultraungu yang terbaik. Kaedah pengekstrakan
cecair-cecair (LLE) dan pengekstrakan fasa pepejal (SPE) juga dijalankan untuk
menilai kaedah pengekstrakan terbaik bagi antosianin daripada sampel plasma
tikus. Kesan mempelbagaikan jenis dan peratusan pelarut organik, jenis dan
kepekatan larutan penimbal, kadar aliran fasa bergerak, suhu turus dan panjang
gelombang pengesan ultraungu telah diuji. Kaedah pengoptimuman kromatografi
menunjukkan komposisi fasa bergerak bagi larutan akueus asid trifluoroasettik
0.1% dan asetonitril dalam nisbah 81:19, dengan kadar aliran 0.5 mL/min, pada
suhu turus 30°C dan panjang gelombang pengesanan 525 nm adalah sesuai untuk
analisis sianidin-3-O-glukosida. Kaedah SPE dipilih sebagai kaedah pengekstrakan
terbaik kerana ia menghasilkan puncak kromatogram yang lebih baik berbanding
kaedah LLE. Kesimpulannya, kaedah RRLC yang dibangunkan dalam kajian ini boleh
digunakan untuk menentukan sianidin-3-O-glukosida dalam plasma tikus.
Kata
kunci: kromatografi cecair resolusi pantas, pembangunan kaedah,
sianidin-3-O-glukosida, plasma tikus
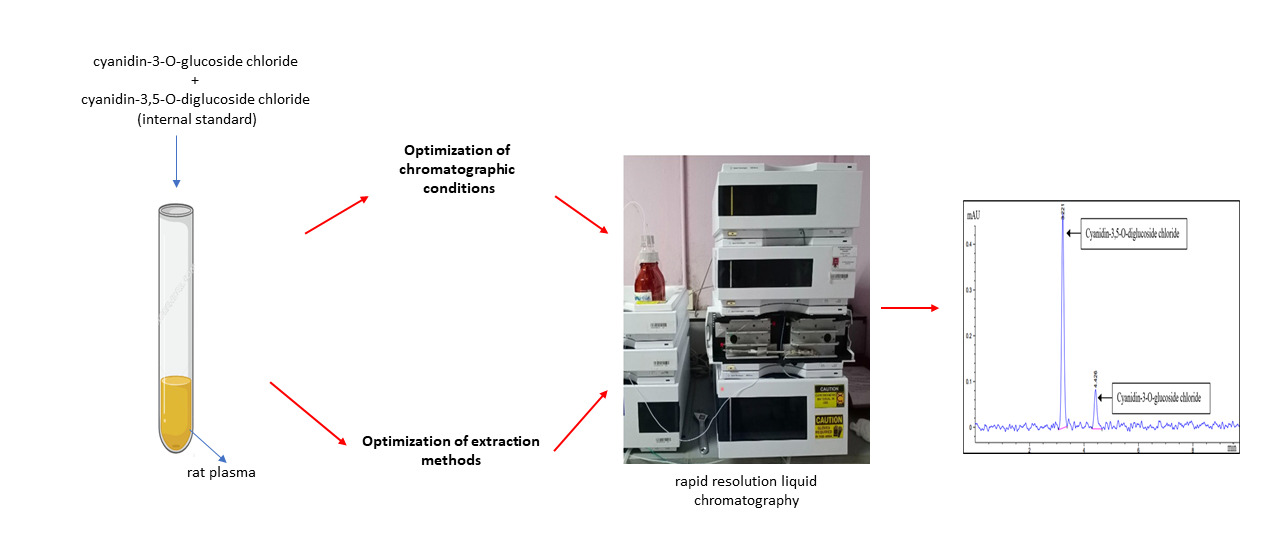
Graphical Abstract
References
1.
Wallace, T. C. and Giusti, M. M. (2015). Anthocyanins. Advances
in Nutrition, 6(5): 619-622.
2.
Passeri, V., Koes, R. and Quattrocchio, F. (2016). New
challenges for the design of high value plant products: stabilization of
anthocyanins in plant vacuoles. Front Plant Science, 7: 1-9.
3.
Riaz, G. and Chopra, R. (2018). A review on
phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomedicine
& Pharmacotherapy, 102: 575-586.
4.
Rubinskiene, M., Jasutiene, I., Venskutonis, P. R. and
Viskelis, P. (2005). HPLC determination of the composition and stability of
blackcurrant anthocyanins. Journal of Chromatographic Science, 43(9):
478-482.
5.
Hapsari, B. W., Manikharda and Setyaningsih, W. (2021).
Methodologies in the analysis of phenolic compounds in roselle (Hibiscus
sabdariffa L.): Composition, biological activity, and beneficial effects on
human health. Horticulturae, 7(2): 1-36.
6.
Angelika, G.-H., Frank, M., & Gotenfels, C. (2009).
Agilent 1200 series rapid resolution LC and rapid resolution LC/MS optimization
guide. Access from https://www.agilent.com/cs/library/ usermanuals/public/1200SeriesRRLC-Optimize
Guide_ebook.pdf. [Access online 11 July 2021].
7.
Banaszewski, K., Park, E., Edirisinghe, I., Cappozzo,
J. C. and Burton-Freeman, B. M. (2013). A pilot study to investigate
bioavailability of strawberry anthocyanins and characterize postprandial plasma
polyphenols absorption patterns by Q-TOF LC/MS in humans. Journal of Berry
Research, 3(2): 113-126.
8.
Harada, K., Kano, M., Takayanagi, T., Yamakawa, O. and
Ishikawa, F. (2004). Absorption of acylated anthocyanins in rats and humans
after ingesting an extract of Ipomoea batatas purple sweet potato tuber.
Bioscience, Biotechnology and Biochemistry, 68(7): 1500-1507.
9.
Saha, S., Singh, J., Paul, A., Sarkar, R., Khan, Z. and
Banerjee, K. (2021). Anthocyanin profiling using UV-Vis spectroscopy and liquid
chromatography mass spectrometry. Journal of AOAC International, 103
(1): 23-39.
10. Dwilistiani,
D., Darwis, D. and Santoni, A. (2015). Characterization of cyanidin
3-(6-acetylglucoside)-5-(3”-coumaryl-6”- malonylglucoside) compound from
cinnamon bud leaves (Cinnamomum burmanni (Ness & T. Ness) Blume) by
HPLC-DAD-ESI-MS. Journal of Chemical and Pharmaceutical Research, 7
(47): 519-523.
11. Nuryanti,
S., Matsjeh, S., Anwar, C. and Raharjo, T. J. (2012). Isolation anthocyanin
from roselle petals (Hibiscus sabdariffa L) and the effect of light on
the stability. Indonesian Journal of Chemistry, 12(2): 167-171.
12. Prior,
R. L. and Wu, X. (2012). Analysis methods of anthocyanins. In Z. Xu & L. R.
Howard (Eds.), Analysis of Antioxidant-Rich Phytochemicals, John Wiley
& Sons, New Jersey: pp. 149-180.
13. Durst,
R. W. and Wrolstad, R. E. (2005). Separation and characterization of
anthocyanins by HPLC. In Current Protocols in Food Analytical Chemistry,
John Wiley & Sons, New Jersey: pp. 33-45.
14. Deineka,
V. I., Deineka, L. A. and Saenko, I. I. (2015). Regularities of anthocyanins
retention in RP HPLC for “water–acetonitrile–phosphoric acid” mobile phases. Journal
of Analytical Methods in Chemistry, 2015 (2015): 1-6.
15. Guzzetta,
A. (2001). Reverse phase HPLC basics for LC/MS. Access from http:// www.ionsource.com/tutorial/chromatography/
rphplc.htm.
[Access online 18 July 2021].
16. Ukić,
Š., Rogošić, M., Novak, M., Šimović, E., Tišler, V. and Bolanča,
T. (2013). Optimization of IC separation based on isocratic-to-gradient
retention modeling in combination with sequential searching or evolutionary
algorithm. Journal of Analytical Methods in Chemistry, 2013 (1): 1-11.
17. Gilar,
M., Jaworski, A. and McDonald, T. S. (2014). Solvent selectivity and strength
in reversed-phase liquid chromatography separation of peptides. Journal of
Chromatography A, 1337(1): 140-146.
18. Chua,
Y. A., Abdullah, W. Z., Yusof, Z. and Gan, S. H. (2019). Validation of HPLC and
liquid-liquid extraction methods for warfarin detection in human plasma and its
application to a pharmacokinetics study. ASM Science Journal, 12(2019):
1-10.
19. Yabré,
M., Ferey, L., Somé, I. T. and Gaudin, K. (2018). Greening reversed-phase
liquid chromatography methods using alternative solvents for pharmaceutical
analysis. Molecules : A Journal of Synthetic Chemistry and Natural
Product Chemistry, 23(5): 1065-1089.
20. Afsah-Hejri,
L., Jinap, S., Arzandeh, S. and Mirhosseini, H. (2011). Optimization of HPLC
conditions for quantitative analysis of aflatoxins in contaminated peanut. Food
Control, 22(3–4): 381-388.
21. Dolan,
J. W. (2002). The importance of temperature. LC GC Europe, 20(6):
524-530.
22. Chua,
Y. A., Abdullah, W. Z. and Gan, S. H. (2012). Development of a high-performance
liquid chromatography method for warfarin detection in human plasma. Turkish
Journal of Medical Sciences, 42 (5): 930-941.
23. Liu,
Y., Liu, Y., Tao, C., Liu, M., Pan, Y. and Lv, Z. (2018). Effect of temperature
and pH on stability of anthocyanin obtained from blueberry. Journal of Food
Measurement and Characterization, 12(3): 1744- 1753.
24. Martín,
J., Navas, M. J., Jiménez-Moreno, A. M. and Asuero, A. G. (2017). Anthocyanin
pigments: Importance, sample preparation and extraction. In M. Soto-Hernandez,
M. Palma-Tenango and M. R. Garcia-Mateos (Eds.), Phenolic Compounds -
Natural Sources, Importance and Applications, Intech Open, London: pp.
117-152.
25. Garcia-Salas,
P., Morales-Soto, A., Segura-Carretero, A. and Fernández-Gutiérrez, A. (2010).
Phenolic-Compound-extraction systems for fruit and vegetable samples. Molecules,
15(12): 8813-8826.
26. Crawford
Scientific. (2017). Peak tailing in HPLC. Access from https://www.crawfordscientific.com/
chromatography-blog/post/peak-tailing-in-hplc. [Access online 19 July 2021].
27. Denoulet,
B. (2020). The perfect peak shape: Five solutions to peak tailing problems.
Access from https://www.barts-blog.net/the-perfect-peak-shape-five-solutions-to-peak-tailing-problems/.[Access
online 19 July 2021].