Malaysian
Journal of Analytical Sciences, Vol 26 No 6 (2022): 1249 - 1259
MODELING
THE TIME RESPONSE OF CURRENT USING INTERDIGITATED ARRAYS IN A SHALLOW
ELECTROCHEMICAL
CELL
(Pemodelan Respons Arus Mengunakan
Susunan Interdigital dalam Sel Elektrokimia Cetek)
Cristian F. Guajardo
Yévenes1,2,* and Werasak Surareungchai3,4
1Biological Engineering
Program, Faculty of Engineering,
2Pilot Plant Development and
Training Institute,
3Nanoscience and Nanotechnology
Graduate Program,
4School of Bioresources and
Technology,
King Mongkut’s University of Technology Thonburi,
49 Soi Thian Thale 25, Thanon Bang Khun Thian Chai Thale
Bangkok 10150, Thailand
*Corresponding author: cristian.gua@kmutt.ac.th
Received: 9 February 2022; Accepted: 25
July 2022; Published: 27 December 2022
Abstract
The interdigitated array of electrodes (IDAE) is a
common choice for integration in small electrochemical sensors due to the
amplified currents and fast response times. The design and assessment of IDAE
performance in microfluidic cells require the use of mathematical models, in
which the diffusion equation is widely applied. Analytical solutions for this
equation are available for IDAEs in tall cells; however, they are not currently
available for shallow cells, as is the case with microfluidic devices. The issue
of whether it is possible to model the time response of IDAEs in shallow cells
using simple exponential functions is addressed in this work. This is achieved
by numerically solving the diffusion equation to obtain the time response of
the current and later by applying non-linear regression to obtain exponential
models. The current response is generally complex due to the many Fourier
harmonics involved. However, the findings reveal that when the elapsed time is
greater than some characteristic time, the current response can be approximated
by an exponential curve. Furthermore, higher currents can be obtained at the
expense of longer response times when the cell is tall, while shorter response
times can be obtained at the expense of lower currents when the cell is
shallow.
Keywords: Shallow electrochemical cell, Interdigitated
arrays, Numerical simulation, Current response.
Abstrak
Elektrod
susunan interdigital (IDEA) adalah pilihan biasa bagi intergrasi dalam sensor
elektrokimia kecil berdasarkan arus teramplikasi dan respons masa yang pantas.
Rekabentuk dan penilaian bagi prestasi IDEA di dalam sel mikrobendalir
memerlukan pemodelan matematik, di mana persamaan resapan digunakan secara
meluas. Penyelesaian analitikal bagi persamaan ini sesuai bagi IDEA dalam sel
tinggi; namun belum ada bagi sel cetek, khusus bagi kes bersama mikrobendalir. Isu terhadap kebarangkalian ia
berhasil terhadap model respons masa di dalam sel cetek menggunakan fungsi
eksponen mudah telah dikaji. Ia dicapai
melalui penyelesaian berangka persamaan resapan untuk mendapatkan respons masa
bagi arus dan kemudian digunakan bagi regresi tak linear untuk mendapatkan
model eksponen. Respons arus secara umum adalah
kompleks disebabkan oleh penglibatan harmonik Fourier. Namun, hasil kajian
mendapati apabila masa berlalu lebih kuat berbanding masa pencirian, responsa
arus boleh dianggar oleh lengkung eksponen. Selanjutnya, arus yang tinggi boleh
diperolehi pada masa respons yang lebih lama apabila susunan sel adalah tinggi,
sebaliknya masa respons yang lebih pendek diperolehi pada arus yang rendah di
mana kedudukan sel adalah cetek.
Kata kunci: sel elektrokimia cetek, susunan interdigital, simulasi
berangka, respons arus
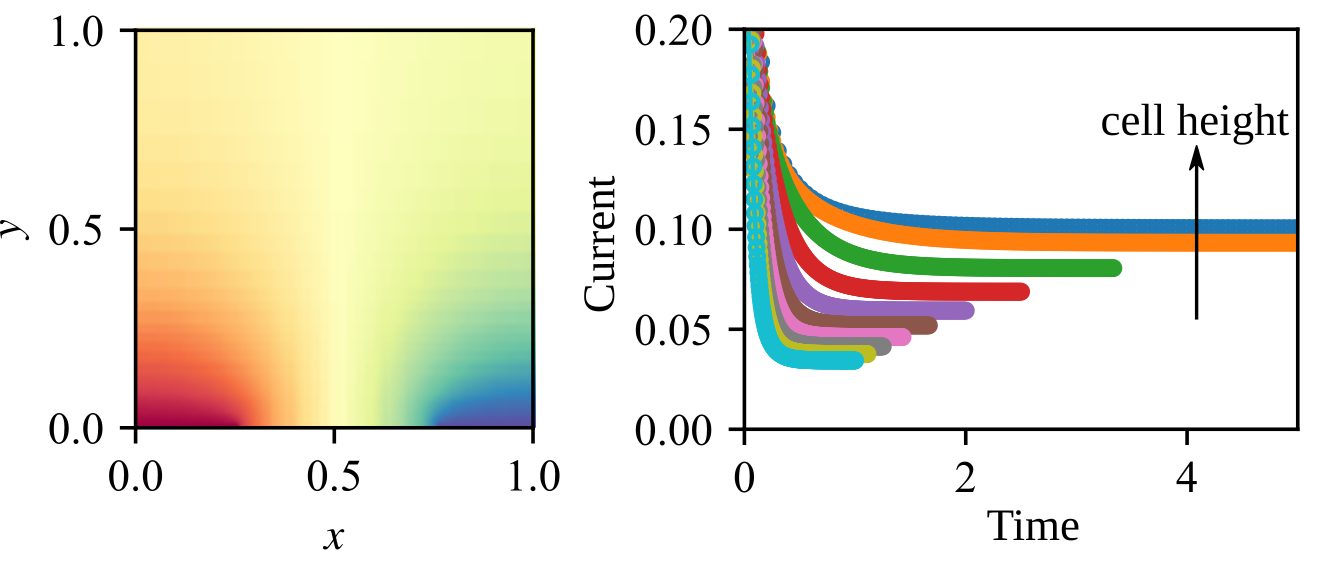
Graphical Abstract
References
1.
Karimian,
N. and Ugo, P. (2019). Recent advances in sensing and biosensing with arrays of
nanoelectrodes. Current Opinion in Electrochemistry, 16: 106-116.
2.
Rackus,
D. G., Shamsi, M. H. and Wheeler, A. R. (2015). Electrochemistry, biosensors
and microfluidics: A convergence of fields. Chemical Society Reviews,
44: 5320-5340.
3.
Gencoglu,
A. and Minerick, A. R. (2014). Electrochemical
detection techniques in micro- and nanofluidic devices. Microfluidics and Nanofluidics, 17: 781-807.
4.
Aoki,
K., Morita, M., Niwa, O. and Tabei, H. (1988). Quantitative analysis of
reversible diffusion-controlled currents of redox soluble species at
interdigitatedgitated array electrodes under steady-state conditions. Journal
of Electroanalytical Chemistry and Interfacial Electrochemistry, 256. 269-282.
5.
Kanno,
Y., Goto, T., Ino, K., Inoue, K. Y., Takahashi, Y., Shiku, H. and Matsue, T.
(2014). SU-8-based flexible amperometric device with IDA electrodes to
regenerate redox species in small spaces. Analytical Sciences, 30:
305-309.
6.
Heo,
J.-I., Lim, Y. and Shin, H. (2013). The effect of channel height and electrode
aspect ratio on redox cycling at carbon interdigitated array nanoelectrodes confined
in a microchannel. Analyst, 138: 6404-6411.
7.
Oldham,
K. and Myland, J. (1994). Fundamentals of electrochemical science. Academic
Press, UK. §5:8.
8.
Guajardo,
C., Ngamchana, S. and Surareungchai, W. (2013). Mathematical modeling of
interdigitated electrode arrays in finite electrochemical cells. Journal of
Electroanalytical Chemistry, 705: 19-29.
9.
Morf,
W. E., Koudelka-Hep, M., de Rooij, N. F. (2006). Theoretical treatment and
computer simulation of microelectrode arrays. Journal of Electroanalytical
Chemistry, 590: 47-56.
10. Britz, D. and Strutwolf, J.
(2016). Digital simulation in electrochemistry. Springer International
Publishing, Switzerland. §7.2.
11. Guyer, J. E., Wheeler, D. and Warren, J. A. (2009).
FiPy: Partial differential equations with Python.
Computing in Science and Engineering, 11(3): 6-5.
12.
Guajardo
Y., Cristian F. and Surareungchai, W. (2021).
Simulations for the current’s time response of interdigitated arrays in shallow
electrochemical cells using FiPy.
https://doi.org/10.5281/zenodo.5633322 [software].