Graphical
Abstract
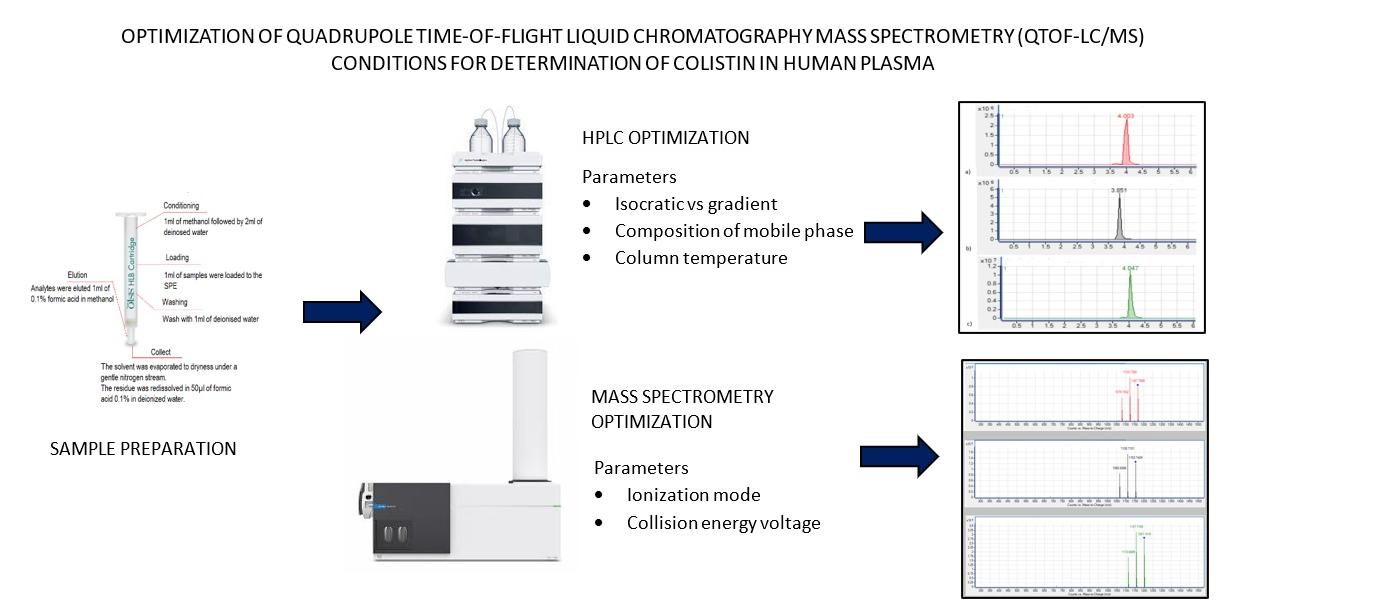
References
1. Tsuji, B. T., Pogue, J, M., Zavascki,
A. P., Paul, M., Daikos, G, L., Forrest, A., Giacobbe, D, R., Viscoli C., Giamarellou, H., Karaiskos, I.,
Kaye, D., Mouton, J. W., Tam, V. H., Thamlikitkul,
V., Wunderink, R. G., Li, J., Nation, R. L. and Kaye,
K. S. (2019). international consensus guidelines
for the optimal use of the polymyxins: Endorsed by the American College of
Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and
Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDS). Pharmacotherapy,
39 (1): 10-39.
2. Nation, R., L., Garonzik, S. M.,
Thamlikitkul, V., Giamarellos-Bourboulis, E. J., Forrest, A., Paterson, D. L.,
Li, J. and Silveira, F. P. (2017). Dosing guidance for intravenous colistin in
critically ill patients. Clinical Infectious Diseases, 64 (5): 565-571.
3. Ehrentraut, S. F., Muenster, S., Kreyer,
S., Theuerkauf, N. U., Bode, C., Steinhagen, F., Ehrentraut, H., Schewe, J. C.,
Weber, M., Putensen, C. and Muders,
T. (2020). Extensive therapeutic drug monitoring of colistin
in critically Ill patients reveals undetected risks. Microorganisms,
8(3): 415.
4. Bergen, P. J., Li, J., Rayner, C. R. and
Nation, R. L. (2006). Colistin methanesulfonate is an inactive prodrug of
colistin against Pseudomonas aeruginosa. Antimicrobial Agents
Chemotheraphy, 50(6): 1953-1958. 5. Li,
J., Nation, R. L., Turnidge, J. D., Milne, R. W., Coulthard, K., Rayner, C. R.
and Paterson, D. L. (2006). Colistin: the re-emerging antibiotic for
multidrug-resistant Gram-negative bacterial infections. Lancet Infectious
Disease, 6(9): 589-601.
6. Milne, R. W., Nation, R. L., Li, J.,
Coulthard, K. and Turnidge, J. D. (2003). Stability of colistin and colistin
methanesulfonate in aqueous media and plasma as determined by high-performance
liquid chromatography. Antimicrobial Agents Chemotherapy, 47(4):
1364-1370.
7. Dudhani, R. V., Nation, R. L. and Li, J.
(2010). Evaluating the stability of colistin and colistin methanesulphonate in
human plasma under different conditions of storage. Journal of Antimicrobial
Chemotherapy, 65(7): 1412-1415.
8. Orwa, J. A., Govaerts, C., Gevers, K.,
Roets, E., Van Schepdael, A. and Hoogmartens, J. (2002). Study of the stability
of polymyxins B(1), E(1) and E(2) in aqueous solution using liquid
chromatography and mass spectrometry. Journal of Pharmaceutical Biomedical
Analysis, 29(1-2): 2003-2012.
9. Karvanen, M., Malmberg, C., Lagerback, P.,
Friberg, L. E. and Cars, O. (2017). Colistin is extensively lost during
standard in vitro experimental conditions. Antimicrobial Agents Chemotherapy,
61(11): e00857-17.
10. Li, J., Milne, R. W., Nation, R. L.,
Turnidge, J. D., Coulthard, K. and Valentine, J. (2002). Simple method for
assaying colistin methanesulfonate in plasma and urine using high-performance
liquid chromatography. Antimicrobial Agents Chemotheraphy, 46(10):
3304-3307.
11. Gikas, E., Bazoti, F. N., Katsimardou, M.,
Anagnostopoulos, D., Papanikolaou, K., Inglezos, I., Skoutelis, A., Daikos, G.
L. and Tsarbopoulos, A. (2013). Determination of colistin A and colistin B in
human plasma by UPLC–ESI high resolution tandem MS: Application to a
pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis,
83: 228-236.
12. Gobin, P., Lemaître, F., Marchand, S., Couet,
W. and Olivier, J. C. (2010). Assay of colistin and colistin methanesulfonate
in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrobial
Agents Chemotherapy, 54(5): 1941-1948.
13. Cangemi, G., Barco, S., Castagnola, E.,
Tripodi, G., Favata, F. and D’Avolio, A. (2016). Development and validation of
UHPLC–MS/MS methods for the quantification of colistin in plasma and dried
plasma spots. Journal of
Pharmaceutical and Biomedical Analysis, 129: 1-7.
14. Chepyala, D.,Tsai, I. L., Sun, H. Y., Lin, S.
W. and Kuo, C. H. (2015). Development and validation of a high-performance
liquid chromatography-fluorescence detection method for the accurate
quantification of colistin in human plasma. Journal of Chromatography B,
980: 48-54.
15. Milne, R.W. (2019). Bioanalysis and stability
of polymyxins. polymyxin antibiotics: From labarotory bench to bedside,
advances in experimental medicine and biology. Springer Nature, Switzerland:
pp. 73-87.
16. Li, J., Milne, R. W., Nation, R. L.,
Turnidge, J. D., Coulthard, K. and Johnson, D. W. (2001). A simple method for
the assay of colistin in human plasma, using pre-column derivatization with
9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and
reversed-phase high-performance liquid chromatography. Journal of
Chromatography B, 761(2): 167-175.
17. Le Brun, P. P., de Graaf, A. I. and Vinks, A.
A. (2000). High-performance liquid chromatographic method for the determination
of colistin in serum. Therapeutic Drug Monitoring, 22(5): 589-593.
18. Reed, M. D., Stern, R. C., O’Riordan, M. A.
and Blumer, J. L. (2001). The pharmacokinetics of colistin in patients with
cystic fibrosis. Journal of Clinical Pharmacology, 41 (6): 645-654.
19. Bihan, K., Lu, Q., Enjalbert, M., Apparuit,
M., Langeron, O., Rouby, J., Funck-Brentano, C. and Zahr, N. (2016).
Determination of colistin and colistimethate levels in human plasma and urine
by high-performance liquid chromatography-tandem mass spectrometry. Therapeutic
Drug Monitoring, 38(6): 796–803
20. Jansson, B., Karvanen, M. Cars, O.,
Plachouras, D. and Friberg, L. E. (2009). Quantitative analysis of colistin A
and colistin B in plasma and culture medium using a simple precipitation step
followed by LC/MS/MS. Journal and Pharmaceutical and Biomedical Analysis,
49(3): 760-767.
21. Ma, Z., Wang, J., Gerber, J. P. and Milne, R.
W. (2008). Determination of colistin in human plasma, urine and other
biological samples using LC-MS/MS. Journal of Chromatography B, 862
(1-2): 205-212.
22. Leporati, M., Bua, R. O., Mariano, F.,
Carignano, P., Stella, M., Biancone, L. and Vincenti, M. (2014). Determination
by LC-MS/MS of colistins A and B in plasma and ultrafiltrate from critically
ill patients undergoing continuous venovenous hemodiafiltration. Therapeutic
Drug Monitoring, 36(2): 182-191.
23. Tsai, I. L., Sun, H. Y., Chen, G. Y., Lin, S.
W. and Kuo, C. H. (2013). Simultaneous
quantification of antimicrobial agents for multidrug-resistant bacterial
infections in human plasma by ultra-high-pressure liquid chromatography-tandem
mass spectrometry. Talanta, 116: 593-603.
24. Wan, E. C., Ho, C., Sin, D. W. and Wong, Y.
(2006). Detection of residual bacitracin A, colistin A, and colistin B in milk
and animal tissues by liquid chromatography tandem mass spectrometry. Analytical
and Bioanalytical Chemistry, 385(1): 181-188.
25. Mallet, C. R., Lu, Z. and Mazzeo, J. R.
(2004). A study of ion suppression effects in electrospray ionization from
mobile phase additives and solid-phase extracts. Rapid Communications in
Mass Spectrometry, 18 (1): 49-58.
26. George, R., Haywood, A., Khan, S.,
Radovanovic, M., Simmonds, J. and Norris, R. (2018). Enhancement and
suppression of ionization in drug analysis using HPLC-MS/MS in support of
therapeutic drug monitoring: A review of current knowledge of its minimization
and assessment. Therapeutic Drug Monitoring, 40 (1): 1-8.
27. Sargent, M., (2013). Guide to achieving
reliable quantitative LC-MS measurements. RSC Analytical Methods
Committee. United Kingdom. pp. 35-42.
28. Hanai, Y., Matsuo, K., Kosugi, T., Kusano, A.,
Ohashi, H., Kimura, I., Hirayama, S., Nanjo, Y., Ishii, Y., Sato, T., Miyazaki,
T., Nishizawa, K and Yoshio, T. (2018). Rapid, simple, and clinically
applicable high-performance liquid chromatography method for clinical
determination of plasma colistin concentrations. Journal of Pharmaceutical
Health Care and Sciences, 4: 1-9.
29. Adaway, J. E. and Keevil, B. G. (2012).
Therapeutic drug monitoring and LC–MS/MS. Journal of Chromatography B,
883–884: 33–49.
30. Dotsikas, Y., Markopoulou, C. K., Koundourellis,
J. E. and Loukas, Y. L. (2011). Validation of a novel LC-MS/MS method for the
quantitation of colistin A and B in human plasma. Journal of Separation
Science, 34(1): 37-45.
31. Food and Drug Administration (2018). Bioanalytical
method validation guidance. Access from
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry.
[Access online 29 April 2020].