SURFACTANT FUNCTIONALIZATION OF MAGNETIC NANOPARTICLES:
A STUDY ON THE PARTICLE SIZE DISTRIBUTION AND ZETA POTENTIAL AS A NANOFLUID TO
IMPROVE THERMAL CONDUCTIVITY
(Perfungsian Surfaktan Nanopartikel Magnet:
Kajian Mengenai Taburan Saiz Zarah dan Potensi Zeta Sebagai Bendalir Nano untuk
Meningkatkan Kekonduksian Terma)
Cheah Ching Wei1,
Noorashikin Md. Saleh1*, Nik Nur Atiqah Nik Wee1, Beh
Shiuan Yih1,
Ahmad Fazlizan2,
Farhanini Yusoff3
1,2Department of Chemical and Process Engineering,
Faculty of Engineering
and Built Environment, Universiti Kebangsaan Malaysia,
43600 UKM Bangi,
Selangor, Malaysia
2Institute
of Solar Energy,
Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor,
Malaysia
3Faculty of Science and Marine Environment,
Universiti Malaysia
Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia
*Corresponding author: noorashikin@ukm.edu.my
Received: 18 May 2022; Accepted: 15
September 2022; Published: 27 December
2022
Abstract
Solar
energy has gained increasing popularity in the last decade, resulting in the
development of photovoltaic/thermal (PV/T) systems to improve photovoltaic cell
efficiency. Working fluid is one of the most important components in a heat
transfer system. In this study, Fe3O4@Sylgard 309
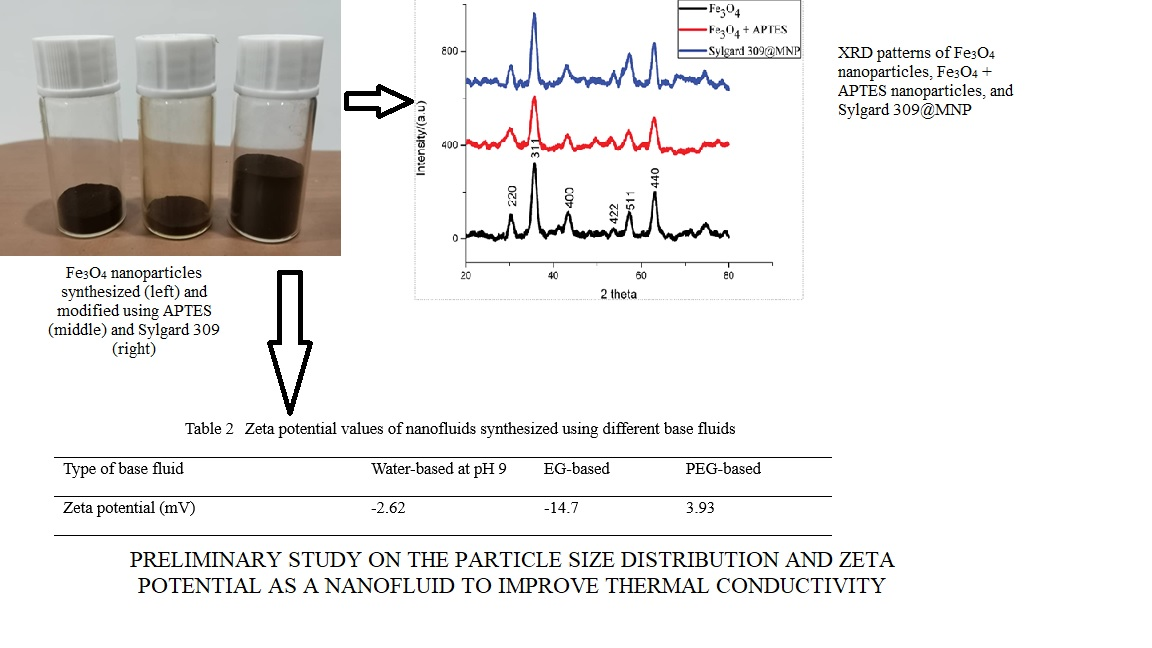
nanoparticles were synthesized for nanofluid preparation. The stability and particle
size of the nanofluid were analyzed using the dynamic light scattering method.
Water-based nanofluid at pH 7 had the highest zeta potential compared to the
same water-based nanofluid at different pHs ranging
from 5.2 to 9.4. The nanofluid synthesized using cetyltrimethylammonium
ammonium bromide and sodium dodecyl sulfate as the surfactant achieved better
stability than the nanofluid synthesized without the addition of the
surfactant. Ethylene glycol (EG)-based nanofluid had the highest zeta potential
value of -2.54 mV compared to the water-based and polyethylene glycol
(PEG)-based nanofluids. Nanoparticles in the PEG-based nanofluid remained
suspended for two days and were better than the water-based and EG-based
nanofluids. The improvement of the thermal conductivity of the metal oxide
nanofluid compared to the conventional base fluid in previous studies was
reviewed. The results showed that the thermal conductivity of nanofluid was
higher than the base fluid up to 49.4%, indicating the potential for nanofluid
applications in PV/T systems if the stability of the nanofluid can be improved.
Keywords: Ferum(III) oxide, magnetic nanoparticle, nanofluid,
thermal conductivity, thermophysical property
Abstrak
Populariti tenaga suria semakin
meningkat sejak sedekad yang lalu dan menghasilkan pembangunan sistem
fotovolta/terma (PV/T) untuk meningkatkan kecekapan sel fotovolta. Bendalir
kerja adalah salah satu komponen terpenting dalam sistem pemindahan haba. Dalam
kajian ini, nanozarah Fe3O4@Sylgard 309 telah disintesis
untuk menyediakan cecair nano. Kestabilan dan saiz zarah cecair nano dianalisis
menggunakan kaedah penyerakan cahaya dinamik. Bendalir nano berasaskan air pada
pH 7 mempunyai potensi zeta yang paling tinggi berbanding dengan cecair nano berasaskan
air yang sama pada pH berbeza antara 5.2 hingga 9.4. Kestabilan cecair nano
yang disintesis menggunakan setiltrimetilammonium bromida dan natrium dodesil
sulfat sebagai surfaktan adalah lebih baik iaitu -2.54 mV daripada cecair nano
yang disintesis tanpa penambahan surfaktan. Bendalir nano berasaskan etilena
glikol (EG) mempunyai nilai potensi zeta yang paling tinggi berbanding dengan
cecair nano berasaskan air dan polietilena glikol (PEG). Nanozarah dalam cecair
nano berasaskan PEG kekal terampai selama dua hari dan lebih baik daripada
cecair nano berasaskan air dan EG. Peningkatan kekonduksian terma bagi cecair
nano oksida logam berbanding dengan cecair asas konvensional dalam kajian
terdahulu telah dikaji semula. Keputusan menunjukkan bahawa kekonduksian terma
cecair nano adalah lebih tinggi daripada cecair asas sehingga 49.4% dan
mempunyai potensi aplikasi cecair nano dalam sistem PV/T sekiranya kestabilan
cecair nano dapat dipertingkatkan.
Kata
kunci: Ferum(III) oksida, nanozarah
magnet, bendalir nano, termal kekonduksian, sifat termofizik
Graphical
Abstract
References
1.
IEA
(2019). Global Energy & CO2
Status Report. 2019
2.
Ritchie,
H., and Roser, M. (2017). CO2 and
greenhouse gas emissions. Renewable
Energy. 2017: pp. 1-10.
3.
Dahlan,
N. Y., and Ismail, M. F. A (2015). Review on solar thermal technologies for low
and medium temperature industrial process heat. Journal of Industrial Technology, 23(1): 25-44.
4.
Ibrahim,
A., and El-Amin, A. (2012). Temperature effect on the performance of n-type
c-Si film grown by linear facing target sputtering for thin film silicon
photovoltaic devices. Optoelectronics and
Advanced Materials-Rapid Communications, 6:73-77.
5.
Jama,
M., Singh, T., Gamaleldin, S. M., Koc,
M., Samara, A., Isaifan, R. J. (2016). Critical
review on nanofluids. Journal of
Nanomaterials, 26:
6717624.
6.
Al-Shamani, A. N., Sopian, K., Mat,
S., Hasan, H. A., Abed, A. M., and Ruslan, M. (2016). Experimental studies of
rectangular tube absorber photovoltaic thermal collector with various types of
nanofluids under the tropical climate conditions. Energy Conversion and Management, 124: 528-542.
7.
Al-Waeli, A. H., Sopian, K., Chaichan, M. T., Kazem, H. A., Hasan, H. A., and Al-Shamani, A. N. (2017). An experimental investigation of SiC nanofluid as a base-fluid for a photovoltaic thermal
PV/T system. Energy Conversion and
Management, 142, 547-558.
8.
Said,
Z., Sabiha, M., Saidur, R.,
Hepbasli, A., Rahim, N. A., Mekhilef,
S., et al. (2015). Performance enhancement of a flat plate solar collector
using titanium dioxide nanofluid and polyethylene glycol dispersant. Journal of Cleaner Production, 92:
343-353.
9.
Yusoff, M. M., Yahaya, N., Saleh, N.
M., and Raoov, M. (2018). A study on the removal of
propyl, butyl, and benzyl parabens via newly synthesised
ionic liquid loaded magnetically confined polymeric mesoporous adsorbent. RSC Advances, 8(45): 25617-25635.
10.
Guo, T., Lin, M., Huang, J., Zhou, C., Tian, W., Yu,
H., et al. (2018). The recent advances of magnetic
nanoparticles in medicine. Journal of
Nanomaterials, 2018:
7805147.
11.
Noorashikin,
M.S., Mohamad, S. and Abas, M. R.
(2014). Extraction of parabens from water samples using cloud point
extraction of non-ionic surfactant with beta-cyclodextrin as modifier. Journal
of Surfactants and Detergents, 17: 747-758.
12.
Yusoff, F., Khing, N. T., Hao, C. C., Sang, L. P., Muhamad, N. A. B.,
and Saleh, N. M. (2018). The electrochemical behavior of zinc oxide/reduced
graphene oxide composite electrode in dopamine. Malaysian Journal of Analytical Sciences, 22(2): 227-237.
13.
Noorashikin, M. S., Sohaimi, N. M., Suda, N., Aziz, H. Z., Zaini, S.
R. M., Kandasamy, S., et al. (2017).
The application of cloud point extraction in environmental analysis. Journal Sustainability Science Management, 12:
79-95.
14.
Noorashikin,
M., Mohamad, S., and Abas, M. (2016). Determination of parabens in water
samples by cloud point extraction and aqueous two-phase extraction using
high-performance liquid chromatography. Desalination
and Water Treatment, 57(47): 22353-22361.
15.
Norseyrihan,
M. S., Noorashikin, M. S., Adibah,
M., and Farhanini, Y. (2016). Cloud point extraction
of methylphenol in water samples with low viscosity
of non-ionic surfactant Sylgard 309 coupled with
high-performance liquid chromatography. Separation
Science and Technology, 51(14): 2386-2393.
16.
Ariffin, M. M., Sohaimi, N. M., Yih, B. S., and
Saleh, N. M. (2019). Magnetite nanoparticles coated with surfactant Sylgard 309 and its application as an adsorbent for paraben
extraction from pharmaceutical and water samples. Analytical Methods,
11(32): 4126-4136.
17.
Baker,
I. (2018). Magnetic nanoparticle synthesis. Nanobiomaterials, 2018: 197-229.
18.
Gonzalez-Carreno, T., Morales, M., Gracia,
M., and Serna, C. (1993). Preparation of uniform γ-Fe2O3
particles with nanometer size by spray pyrolysis. Materials Letters, 18(3): 151-155.
19.
Haiza, H., Yaacob, I., and Azhar, A. Z. A. (2018). Thermal conductivity of water-based
magnetite ferrofluids at different temperature for heat transfer applications. Solid State Phenomena, 280: 36-42.
20.
Moon,
J.-W., Rawn, C. J., Rondinone, A. J., Love, L. J., Roh, Y., Everett, S. M., et al. (2010). Large-scale
production of magnetic nanoparticles using bacterial fermentation. Journal of Industrial Microbiology &
Biotechnology, 37(10):
1023-1031.
21.
Safee, N. H. A., Abdullah, M.
P., and Othman, M. R. (2010). Carboxymethyl chitosan− Fe3O4
nanoparticles: synthesis and characterization. Malaysian Journal Analytical Sciences, 14: 63-68.
22.
Sugimoto,
T., Wang, Y., Itoh, H., and Muramatsu, A. (1998).
Systematic control of size, shape and internal structure of monodisperse
α-Fe2O3 particles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 134(3):
265-279.
23.
Sun,
S., and Zeng, H. Size-controlled synthesis of magnetite nanoparticles. Journal of the American Chemical Society,
124(28): 8204-8205.
24.
Wang,
X., Zhuang, J., Peng, Q., and Li, Y. (2005). A general strategy for nanocrystal
synthesis. Nature,
437(7055): 121-124.
25.
Zhang,
G., Liao, Y., and Baker, I. (2010). Surface engineering of core/shell iron/iron
oxide nanoparticles from microemulsions for hyperthermia. Materials Science and Engineering, 30(1): 92-97.
26.
Biehl,
P., Von der Lühe, M., Dutz,
S., and Schacher, F. H. (2018). Synthesis,
characterization, and applications of magnetic nanoparticles featuring
polyzwitterionic coatings. Polymers,
10(1): 91.
27.
Ghadiri, M., Sardarabadi, M., Pasandideh-fard,
M., and Moghadam, A. J. (2015). Experimental investigation of a PVT system performance
using nano ferrofluids. Energy Conversion
and Management, 103:
468-476.
28.
Mohanraj,
K., and Sivakumar, G. (2017). Synthesis of γ-Fe2O3,
Fe3O4 and copper doped Fe3O4
nanoparticles by sonochemical method. Sains Malaysiana, 46(10): 1935-1942.
29.
Mufti,
N., Atma, T., Fuad, A., and Sutadji,
E. (2014). Synthesis and characterization of black, red and yellow
nanoparticles pigments from the iron sand. In AIP Conference Proceedings, 1617:
165-169.
30.
Song,
K., Lee, S., Suh, C.-Y., Kim, W., Ko, K.-S., and Shin, D. (2012). Synthesis and
characterization of iron oxide nanoparticles prepared by electrical explosion
of Fe wire in ArO2 gas mixtures. Materials Transactions, M2012186.
31.
Nalle, F., Wahid, R., Wulandari, I., and Sabarudin, A.
(2019). Synthesis and characterization of magnetic Fe3O4
nanoparticles using oleic acid as stabilizing agent. Rasayan Journal of Chemistry, 12(1): 14-21.
32.
Xie, L., Qian, W., Yang,
S., Sun, J., and Gong, T. (2017). A facile and green synthetic route for
preparation of heterostructure Fe3O4@Au nanocomposites. MATEC Web of Conferences, 88:
02001.
33.
Zhou,
J., Meng, L., Lu, Q., Fu, J., and Huang, X. (2009). Superparamagnetic submicro-megranates: Fe3O4
nanoparticles coated with highly cross-linked organic/inorganic hybrids. Chemical Communications, 42: 6370-6372.
34.
Lowry,
G. V., Hill, R. J., Harper, S., Rawle, A. F., Hendren, C. O., Klaessig, F., et al. (2016). Guidance to improve the
scientific value of zeta-potential measurements in nanoEHS.
Environmental Science: Nano,
3(5): 953-965.
35.
Bhattacharjee,
S. (2016). DLS and zeta potentialwhat they are and what they are not?. Journal of
Controlled Release, 235: 337-351.
36.
Ostolska,
I., and Wiśniewska, M. (2014). Application of
the zeta potential measurements to explanation of colloidal Cr2O3
stability mechanism in the presence of the ionic polyamino
acids. Colloid and Polymer Science,
292(10): 2453-2464.
37.
Salopek, B., Krasic, D. and Filipovic, S.
(1992). Measurement and application of zeta-potential. Rudarsko-geolosko-naftni zbornik, 4(1):
147.
38.
Abadeh, A., Passandideh-Fard, M., Maghrebi, M. J. and Mohammadi, M.
(2019). Stability and magnetization of Fe3O4/water
nanofluid preparation characteristics using Taguchi method. Journal of Thermal Analysis and Calorimetry,
135(2): 1323-1334.
39.
Uskoković,
V., Castiglione, Z., Cubas, P., Zhu, L., Li, W. and Habelitz, S. (2010). Zeta-potential and particle size
analysis of human amelogenins. Journal of Dental Research, 89(2): 149-153.
40.
Wang,
T., Ni, M., Luo, Z., Shou, C., and Cen, K. (2012). Viscosity and aggregation
structure of nanocolloidal dispersions. Chinese Science Bulletin,
57(27): 3644-3651.
41.
Ghadimi, A., and Metselaar, I. H. (2013). The influence of surfactant and
ultrasonic processing on improvement of stability, thermal conductivity and
viscosity of titania nanofluid. Experimental
Thermal and Fluid Science, 51: 1-9.
42.
Gensdarmes,
F. (2015). Methods of Detection and Characterization. Nanoengineering, 2015: 55-84.
43.
Wang,
L., Wang, X., Gu, R., Wang, H., Yao, L., Wen, L., et al. (2018). Observations
of fine particulate nitrated phenols in four sites in northern China:
concentrations, source apportionment, and secondary formation. Atmospheric Chemistry and Physics,
18(6): 4349-4359.
44.
Li,
C. H., and Peterson, G. (2006). Experimental investigation of temperature and
volume fraction variations on the effective thermal conductivity of
nanoparticle suspensions (nanofluids). Journal
of Applied Physics, 99(8): 084314.
45.
Şeşen,
M., Tekşen, Y., Şendur,
K., Pınar Mengüç, M., Öztürk, H., Yağcı Acar, H., et al. (2012). Heat transfer enhancement with
actuation of magnetic nanoparticles suspended in a base fluid. Journal of Applied Physics,
112(6): 064320.
46.
Abareshi,
M., Goharshadi, E. K., Zebarjad,
S. M., Fadafan, H. K. and Youssefi,
A. (2010). Fabrication, characterization and measurement of thermal
conductivity of Fe3O4 nanofluids. Journal
of Magnetism and Magnetic Materials, 322(24): 3895-3901.
47.
Yüksel,
N. (2016). The review of some commonly used methods and techniques to measure
the thermal conductivity of insulation materials. In A. Almusaed, & A.
Almssad (Eds.), Insulation Materials in
Context of Sustainability. IntechOpen.
